| Issue |
BIO Web Conf.
Volume 20, 2020
1st International Conference on Tropical Wetland Biodiversity and Conservation (ICWEB 2019)
|
|
|---|---|---|
| Article Number | 04003 | |
| Number of page(s) | 3 | |
| Section | Wildlife Biology and Medicine | |
| DOI | https://doi.org/10.1051/bioconf/20202004003 | |
| Published online | 01 June 2020 | |
Molecular Bird Sexing on Fischeri Lovebird (Agapornis fischeri) by Using Polymerase Chain Reaction
1 Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta. Jl. Fauna 2 Karangmalang, Yogyakarta 55281, Indonesia
2 Research Center for Biology, Indonesian Institute of Sciences (LIPI), Jl Jakarta-Bogor km.46, Cibinong, West Java, Indonesia, 16911
* Corresponding author: arisharyanto@ugm.ac.id
Fischeri Lovebird (Agapornis fischeri) found originally in Africa which has spread to many countries. In Indonesia, Fischeri Lovebird is popular as a pet animal. This lovebird is a monomorphic bird, so it is difficult to differentiate morphologically between male and female birds. In general, a male lovebird has ZZ homozygotes, whereas females' lovebird has ZW heterozygous of their sex chromosome. These sex chromosomes set used as study targets for molecular bird sexing of many species of birds because this method is effective and simple to perform. This method targeted to amplify the Chromodomain Helicase DNA-binding (CHD) gene, which found into the sex chromosome of male and female birds. The objective of this study was to rapid molecular bird sexing of Fischeri Lovebird by using PCR methods. Research samples were collected from feather calamus of A. fischeri. The total sample was 11 feathers from A. fischeri. which were collected three to six feathers for each lovebird. Then the research was followed by DNA extraction from calamus feathers, DNA amplification by PCR and agarose gel electrophoresis of PCR products and visualization of PCR predicts by UV-Transilluminator in darkroom. It concluded that PCR amplification using NP, MP and P2 primers produced double DNA bands in size of 400 bp on Z chromosome and bp on W chromosome for female Fischeri Lovebird, whereas for male Fischeri Lovebird only produced a single DNA band in size of 400 bp on Z chromosome. From eleven samples of Fischeri Lovebird showed a total of five females and six male Fischeri Lovebirds.
© The Authors, published by EDP Sciences, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Lovebird is a common name for small parrots belongs to genus Agapornis, family Psittaculidae, Order Psittaciformes [1]. The bird is about 15 cm long, characterized by a short blunt tail, a relatively large, sharp beak, zygodactyl feet, and have a variety of colors on their upper body, depending on the species. Lovebird is a very vocal, playful, and active bird [2]. In Indonesia, lovebirds are very popular as a pet animal, the result that lovebird breeding is a promising business [3].
Lovebird is divided into two groups, namely the dimorphic and monomorphic groups. Dimorphic meaning there are differences between males and females of the same species, including the Agapornis cana, A.taranta, and A.pullaria. Monomorphic meaning there are no differences between males and females of the same species, including A. swinderniana, A. nigrigenis, A. fischeri, A. personata, A. lilianae, and A.roseicollis [2]. The objective of this study was to rapid molecular bird sexing of Fischeri Lovebird by using PCR methods.
2 Material and Methods
This research was conducted at the Biochemistry Laboratory, Faculty of Veterinary Medicine, Universitas Gadjah Mada. The research samples were feathers from 11 Fischeri Lovebird (A. fischeri) from bird owners and were labeled with AF1 to AF11. Deoxyribonucleic acid (DNA) was isolated from samples based on the Gsync DNA Extraction Kit (Geneaid, Taiwan) protocol, but the incubation time was extended to overnight aimed at making DNA well extracted so that the DNA bands produced when exposed to UV light became clear.
The results of DNA isolation were used as DNA templates in the process of amplification using the PCR method. DNA fragments were amplified by targeting the CHD gene on the sex chromosome using P2, NP, and MP primers. Primary base arrangements and PCR products are presented in Table 1. A mixture of 25 µL of PCR reagents for bird DNA in one reaction consists of 12,5 μl MyTaq™ DNA Polymerase, 1 μl forward primer [10 pmol], 1μl reverse primer [10 pmol], and 9,5 μl isolated DNA. The mixture was put into the PCR machine with controlled temperature and duration of the PCR reaction, beginning with predenaturation of 94°C for 2 minutes, denaturation of 94°C for 20 seconds, annealing 46°C for 30 seconds, the extension of 72°C for 40 seconds, and a final extension at 72°C for 10 minutes. The denaturation, annealing, and extension stages were repeated as many as 40 cycles.
The results of DNA and PCR isolation can be identified by DNA electrophoresis. Ten DNA samples mixed with loading dye were compared with 100 bp hyperladder markers (Bioline, UK) Electrophoresis was done with a 2.5% agarose gel at a voltage of 100 volts and an electric current of 75 amperes for 45 minutes. DNA bands were observed using UV-Transilluminator wavelength of 280 nm.
Nucleotide sequence of primers used in this research and the primer references.
3 Results
Blood and feather can be used as a sample for molecular sexing [9], blood samples contain more DNA than the feathers but need more energy to handle the bird. Improper handling can cause birds stress, even death. Feather as a sample has advantages, such as easier and faster to collect, less stressful on the birds, the storage requirements for samples are simpler and less expensive compared to the blood samples. The quantity of DNA that can be isolated from feathers was not as much as DNA that was isolated from blood, but with consideration of speed, convenience, and minimal risk, feathers were chosen as samples to molecular birds sexing [9].
Researchers stated that the source of DNA in feathers was obtained from the base of the feather calamus which contains many epithelial cells and contained inhibitors, namely keratin, making the extraction process quite difficult [10]. The results of DNA extraction using a kit produce better DNA quality, but by using of kits will increase costs. The kit used for DNA extraction in this study was Gsync DNA Extraction Kit (Geneaid, Taiwan) but the incubation time was extended to overnight so that DNA can be extracted properly. Electrophoresis of DNA isolation products (Figure 1) were visualized under 280 nm UV light with a 100 bp DNA ladder marker produced a luminous DNA fragment due to the presence of SYBRsafe in the samples. DNA bands were seen in samples nine, ten, and eleven. Samples that were empty and did not display DNA bands showed that the DNA in the sample did not exist or very limited quantity, so they cannot be visualized.
Amplification of the CHD gene with primers set of NP, P2, and MP visualized under UV light with a wavelength of 280 nm produced a DNA band for males and two DNA bands for female Fischeri Lovebirds. It caused that birds have sex chromosome composition which different from mammal's sex chromosomes. Heterogametic properties in birds are owned by female birds (ZW) while male birds are homogeneous (ZZ) [11]. The CHD gene could show differences in Z and W alleles in females due to the linkage between the position of the CHD gene and the sex chromosome. The visualization results under UV Transluminator compared to 100 bp hyperladder resulted in a 400 bp PCR product on the Z chromosome and 350 bp on the W chromosome (Figure 2 and Table 2).
Figure 2 showed the electrophoresis results from the PCR products. Lane 1, 3, 4, 6, 7, and 8 there was a single DNA band that indicates that those samples were male Fischeri Lovebird, whereas lanes 2, 5, 9, 10, and 11 showed double DNA bands which means those samples were female Fischeri Lovebirds. This molecular bird sexing on Fischeri Lovebird (Agapornis fischeri) were consistent with the results of molecular bird sexing on other Lovebird species, namely Peach-faced Lovebird (Agapornis roseicollis) reported by researchers who used the same PCR primers set [12].
Table 2 showed the confirmation result of PCR products Lovebird no. 1 to 11. It showed that 6 samples were male Fischeri Lovebirds (AF1, AF3, AF4, AF6, AF7, AF8), while 5 other samples were female Fischeri Lovebirds (AF2, AF5, AF9, AF19. AF11). This result was in line with other researchers, who carried out the molecular bird sexing on some species of captive birds belong to the families of Psittacidae, Cacatuidae, Psittaculidae, Psittrichasiidae, Pelecanidae, Anatidae, Phoenicopteridae, Spheniscidae, Threskiornithidae and Musophagidae [13].
P2 and NP primers were originally designed by Griffith, et al [7], and primer MP was first developed by Ito, et al., [9] to amplify a segment in W sex chromosome only. The P2 and NP were successfully used to differentiate the sex of most bird species, excluding Order Sthrutionioformes [7, 14]. The result of the amplification using NP and P2 primers sets in the Fischeri Lovebird samples showed similar size with the other birds from previous publications [7, 14, 15]. Amplification using NP and MP primers set also showed a similar result with the previous publication [8]. These primers can successfully amplify the segments in W and Z in lovebird’s sex chromosome, hence it can be used to differentiate the sex of the bird.
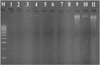 |
Figure 1 Electrophoresis results from total DNA isolation. M= DNA marker (100 bp), 1-11= samples of lovebird. |
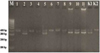 |
Figure 2 Electrophoresis results of CHD gene amplification samples of lovebird. M= DNA marker (100 bp), 1-11= lovebird samples, K1= female control, K2= male control. |
Interpretation of PCR products.
4 Conclusion
It concluded that PCR amplification using NP, MP, and P2 primers produced double DNA bands in the size of 400 bp on Z chromosome and bp on W chromosome for female Fisher Lovebird, whereas for male Fisher Lovebird only produces a single DNA band in size of 400 bp on the Z chromosome. Electrophoresis results of eleven Fisher Lovebird samples showed a total of five females and six male lovebirds. The NP, MP, and P2 primers were successfully used to differentiate the sex of lovebirds.
References
- P. Myers, R. Espinosa, C.S. Parr, T. Jones, G.S. Hammond, and T.A. Dewey. The Animal Diversity Web (online). Accessed at https://animaldiversity.org., (2020) [Google Scholar]
- N. Moustaki. Lovebirds: A Guide to Caring for Your Lovebird. California: Bow Tie Press, (2006) [Google Scholar]
- S. Benjamin and . Yasyifa. Teknik rahasia menciptakan variasi warna lovebird: Dijamin pasti berhasil anti gagal. Jakarta: Lembar Langit Indonesia, (2017) [Google Scholar]
- B. Handono, R. Turut, and S. Gunarso. Lovebird: Sukses menangkarkan dan memelihara. Jakarta: Penebar Swadaya, (2013) [Google Scholar]
- A. Dubiec and M.Z. Neubauer. Molecular technique for sex Identification in birds. Biological Letters 43(1): 3-12, (2006) [Google Scholar]
- I. Khaerunisa, E. Sari, and M. Ulfah, Jakaria, C. Sumantri. Avian sex determination based on Chromo Helicase DNA-binding (CHD) genes using Polymerase Chain Reaction (PCR). Media Peternakan, 36(2): 85-90, (2013) [Google Scholar]
- R. Grifftihs, M.C. Double, K. Orr, and J.G. Dawson. A DNA test to sex most birds. Molecular Ecology, 7: 1071-1075, (1998) [CrossRef] [PubMed] [Google Scholar]
- H. Ito, A. Sudo-Yamaji, M. Abe, T. Murase, and T. Tsubota. Sex identification by alternative polymerase chain reaction methods in Falconiformes. Zoology Science , 20: 339-344, (2003) [CrossRef] [Google Scholar]
- M. Harvey, D. Bonter, L. Stenzler, and I. Lovette. Comparison of plucked feathers versus blood samples as DNA sources for molecular sexing. Journal of Field Orthinology, 77(2), 136-140, (2006) [CrossRef] [Google Scholar]
- C. Hickman, L. Roberts, and F. Hickman. Integrated principles of zoology. Seventh ed. Toronto: Mosby Collage Publishing. 235, (1984) [Google Scholar]
- A.K. Fridolfsson. and H.A. Ellegren. A simple and universal method for molecular sexing of non-ratite birds. Jounal of Avian Biology , 30, 116-121, (1999) [CrossRef] [Google Scholar]
- P. Nugraheni, M. Purwaningrum, R. Widayanti, and A. Haryanto. Sex determination of peach-faced lovebird (Agapornis roseicollis) using polymerase chain reaction (PCR) techniques. IOP Conf. Series: Earth Environ. Sci. 355. 012111, (2019) [Google Scholar]
- M. Purwaningrum, H.A. Nugroho, M. Asvan, K. Karyanti, B. Alviyanto, R. Kusuma, and A. Haryanto. Molecular techniques for sex identification of captive birds. Veterinary World, 12(9): 1506-1513, (2019) [CrossRef] [PubMed] [Google Scholar]
- S. Sulandari and M.S.A. Zein. Application of two molecular sexing methods for Indonesian bird species: Implication for captive breeding programs in Indonesia. Hayati Journal of Biosciences , 19(4): 183-190, (2012) [CrossRef] [Google Scholar]
- H.A. Nugroho and M.S.A. Zein. Evaluasi metode penentuan jenis Kelamin pada Nuri Kepala Hitam (Lorius lory, Linnaeus 1758). Zoo Indonesia, 24(2): 83-93, (2015) [Google Scholar]
All Tables
All Figures
 |
Figure 1 Electrophoresis results from total DNA isolation. M= DNA marker (100 bp), 1-11= samples of lovebird. |
| In the text | |
 |
Figure 2 Electrophoresis results of CHD gene amplification samples of lovebird. M= DNA marker (100 bp), 1-11= lovebird samples, K1= female control, K2= male control. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.



