| Issue |
BIO Web Conf.
Volume 14, 2019
The 12th International Conference on the Health Effects of Incorporated Radionuclides (HEIR 2018)
|
|
|---|---|---|
| Article Number | 02007 | |
| Number of page(s) | 2 | |
| Section | Biokinetics and Bioaccumulation of Radionuclides: Oral presentations | |
| DOI | https://doi.org/10.1051/bioconf/20191402007 | |
| Published online | 07 May 2019 | |
From in vivo to in vitro models to assess bioavailability properties of Plutonium compounds
Laboratoire de Radiotoxicologie, CEA, Paris-Saclay University, 91297 Arpajon - FRANCE
* Corresponding author: anne.vandermeeren@cea.fr
The understanding of the biodistribution of actinides following internal contamination is an important step in the process of dose estimation and risk assessment, as well as in determining an appropriate decorporation treatment. Dose calculation is based, in part, on transferability properties of radiocontaminant. For this purpose ICRP classifies materials according to their absorption from lungs into blood in three types: Fast (F), Moderate (M), and Slow (S). Plutonium (Pu) can exist in multiple forms with differing physico-chemical properties in the work place. It is thus beneficial to develop robust in vitro models as a first approach to predict Pu compound behavior in vivo and to facilitate dose calculation and decisions for appropriate therapy.
For an estimation of Pu biodistribution following inhalation, pulmonary administration in rats is the most common approach. However, animal models remain time consuming and are not appropriate for the study of a great number of compounds. In addition, an a priori knowledge for the categories of a Pu compound involved in a contamination incident would be of great help for an early dose assessment. Therefore, we have developed in vitro models to i) mimic uptake and release by macrophages [1] and ii) determine bioavailability properties of radiocontaminants to predict the in vivo behavior [2].
In the present study, using in vivo and in vitro models, we evaluated the behavior of a Pu compound of unknown physico-chemical properties. Pu solution with unknown properties was intra-tracheally administered in rats, or used for in vitro contamination of macrophages as well as in the acellular model for bioavailability prediction (fig 1). Data were then compared to our historic data obtained using three forms of Pu (colloid, nitrate, citrate) of distinct solubility properties [3] and that we chose to represent the three ICRP transportability classes (respectively S, M and F).
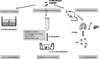 |
Fig.1 Design of the experimental approach |
Two weeks following pulmonary contamination in rats, retention in lungs, bone and liver was evaluated by alpha activity measurement using liquid scintillation counting. Broncho-alveolar lavages (BAL) were carried out to recover alveolar macrophages and quantification of the retained Pu activity. Both the human macrophage-like THP-1 cells and alveolar macrophages from naïve non-exposed rats were exposed to the unknown Pu compound in solution for two hours to assess Pu uptake. Thereafter, Pu-loaded macrophages were cultured in a transwell® culture system and Pu release was measured over 7 days. Finally, in a third experimental approach, agarose gels were prepared so Pu compound solution was entrapped in the static phase representing the retention compartment, and the released fraction in the dynamic phase was an indication of the intrinsic solubility properties of the contaminant.
By comparing the data to previous results obtained in the laboratory using Pu as colloid, nitrate or citrate form, and whatever the model used, we demonstrated that the unknown Pu compound behaved very similarly to Nitrate. Therefore, were able to classify the Pu compound as a type M default absorption type, according to ICRP classification. These results suggest that the in vitro methods could be used as a first approach to predict the biodistribution of a contaminant, and help during the dose assessment process to classify the contaminant according to its solubility properties. In addition, the development and use of in vitro models to predict retention and propose transportability classes fulfill the aims of the 3Rs (Reduce, Refine, Replace) with regard to animal experimentation.
Results presented here were obtained in the frame of the AREVA NC-CEA collaboration.
References
- A. Van der Meeren, A. Moureau, D. Laurent, P. Laroche, JF. Angulo-Mora. Toxicol. in Vitro 37, 25 (2016) [Google Scholar]
- CEA-AREVA NC International Patent Application WO2017109024 published June 29, 2017. [Google Scholar]
- A. Van der Meeren, O. Grémy, D. Renault, A. Miroux, S. Bruel, N. Griffiths, F. Tourdes. J. Radiat. Res. 53, 184 (2012) [CrossRef] [PubMed] [Google Scholar]
© The Authors, published by EDP Sciences, 2019
 This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0 (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0 (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
All Figures
 |
Fig.1 Design of the experimental approach |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.



