| Issue |
BIO Web Conf.
Volume 19, 2020
International Symposium on Indonesian Fauna (ISIF 2019)
|
|
|---|---|---|
| Article Number | 00006 | |
| Number of page(s) | 6 | |
| DOI | https://doi.org/10.1051/bioconf/20201900006 | |
| Published online | 10 April 2020 | |
Unusual call characteristics and acoustic niche adaptation of Limnonectes larvaepartus Iskandar, Evans & Mcguire, 2014 in its habitat (Anura: Dicroglossidae)
1 Museum Zoologicum Bogoriense, Research Center for Biology, Indonesian Istitute of Sciences (LIPI), Widyasatwaloka Building, Jalan Raya Cibinong KM 46, Cibinong, 16911, West Java, Indonesia
2 Adudu Nantu International Conservation Foundation, Gorontalo, Northern Sulawesi, Indonesia
* Corresponding author: hellen.kurniati@gmail.com
The advertisement call of Limnonectes larvaepartus is unique among frogs of the genus Limnonectes which have a true acoustic organ. The calls of three adult males L. larvaepartus were recorded at the foothill of Hutadelita Mountain (N 000 48’ 48.3” E 1220 23’ 00.8”; 396 m asl), Nantu Wildlife Sanctuary, Gorontalo, northern Sulawesi by using an Olympus LS-11 recorder with built-in microphones. Air temperature during recording was around 26°C. Adobe Audition 3.0 software was used to visualize and analyze the calls. The advertisement calls consist of 1-6 pulses, though only one pulse is usually produced. The wave structure of the call of L. laervaepartus is unusual because it is characterized by a short series of pulses that form a pulse train. Each pulse from the beginning of the call until the middle of the call has its own frequency spectrum, whereas the pulse train during the second half of the call becomes tightly compressed and forms one frequency spectrum. Calls of L. larvaepartus do not have a dominant frequency. Minimum frequencies decrease gradually from the beginning to the end of the pulse train; however, the maximum frequency rises gradually. Ascending frequency characters in one pulse also occur in the energy spectrum and bandwidth of frequency. The lowest minimum frequency at the end of pulse train is ~165 Hz; while the highest maximum frequency is also located at the end of the pulse train and is ~6860 Hz. The short pulses call of L. larvaepertus is similar to that of L. hasceanus, although the two species are not close relatives; in L. larvaepartus, the pulse only consists of one period, whereas in L. haschenus it consists of two periods. Considering acoustic adaptation, call of L. larvaepartus has similar frequency spectrum with call of L. modestus, we assume that they would not call at the same time and at the same localities because of their similar acoustic niches.
© The Authors, published by EDP Sciences, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
The genus Limnonectes Fitzinger, 1843 consists of 72 species, 31 of which occur in the Sundaland and Wallacean regions of Indonesia [1]. Sulawesi has five described species of Limnonectes, including L. arathooni (Smith, 1927), L. heinrichi (Ahl, 1933), L. modestus (Boulenger, 1882), L. larvaepartus Iskandar, Evans, & McGuire, 2014 and L. microtympanum (van Kampen, 1909). Molecular phylogenetic studies indicate that the genus Limnonectes contains several undescribed lineages in Thailand [2], on the Malay Peninsula [3], on Sumatra [4], and on Borneo [5]; [6]; [7]. In the Wallacea regions of Sulawesi Island, the genus Limnonectes has adaptively radiated with at least 15 undescribed lineages [8]; [9]; [10]; [11].
Based on our observations of specimens of Limnonectes housed in the Museum Zoologicum Bogoriense (MZB), Sulawesi is the island where mostly of Limnonectes spp have true advertisement calls, due to possession of vocal sacs. Most of Limnonectes species (described and undescribed) that are found on Sulawesi have vocal sacs, except for Limnonectes sp i which is included in L. grunniens group and L. microtympanum. As a comparison between Sulawesi and the large surrounding islands, there is only one species of Limnonectes on Java which has true advertisement calls, L. microdiscus [12], while L. kuhlii produces its call by passing air from its lungs through its buccal cavity because it does not have a vocal sac. In Kalimantan, there are two species that have true advertisement calls, i.e. L. finchi and L. palavanensis [13]; but another species, L. hikidae, which is in the same group with L. kuhlii does not have vocal sac [5]. In Sumatra, only L. microdiscus has true advertisement calls; whereas L. dammermani and L. kadarsani from the Lesser Sunda Islands were found to produce advertisement calls. Among described species of Limnonectes that produce sound in Sulawesi, only two species have described advertisement calls, L. arathooni by [14] and L. modestus by Kurniati & Hamidy [15].
Limnonectes larvaepartus is a very special type of frog with a unique reproductive mode (Figure 1) involving internal fertilization and birth of tadpoles [10]; [16]. The distribution of L. larvaepartus overlaps broadly with L. modestus [10]; [11], with both species found from the northern part of Sulawesi to the western part of central Sulawesi. However, based on microhabitat type, L. modestus inhabits rocky big river with strong water flow, while L. larvaepartus has tendency to inhabit slower side streams and seep areas [10]. Males of L. modestus and L. larvaepartus produce each advertisement calls; with the call of L. modestus already described by Kurniati & Hamidy [15], and the call of L. larvaepartus not yet characterized.
 |
Fig. 1 Adult male of Limnonectes larvaepartus (MZB Amph. 30503) from Nantu Wildlife Sanctuary, Gorontalo, northern Sulawesi (Photograph by A. Hamidy). |
2 Material and Methods
Calls of three adult male L. larvaepartus were recorded on the lower slope of Mount Hutadelita (N 000 48’ 48,3”: E 1220 23’ 00,8”; 396 asl), Nantu Wildlife Sanctuary, Gorontalo, northern Sulawesi (Figure 2). The frogs were recorded on 2 October, 2017 using an Olympus LS-11 recorder with built-in microphones. The recorder was set by using a sampling frequency of 94 kiloHertz (kHz) and a bit rate of 24 bits in WAV format. Air temperature during recording was ~26°C. The length of time for call recording for each individual was about five minutes. Adobe Audition 3.0 software was used to visualize and analyze the calls. Recorded calls were first normalized at -1 decibel (db); due to stereo recording that displayed double visualization of sound wave at the software, then the sound files were converted to 48000 Hz and 16 bits for mono visualization to ensure highest possible recording quality for frequencies up to 24 kHz (https://helpx.adobe.com/audition/using/converting-sample-types.html). Adobe Audition 3.0 software was also used to describe the oscillogram and spectrogram of the call type of L. larvaepartus. Visualization process of sound waves in the form of oscillograms, spectrograms and the dominant frequency were obtained using FFT (Fast Fourier Transformation; 1024 points) using a Hanning window. The terms used in the description of the calls follow Kohler et. al. [17], including pulse duration, call duration, inter-pulse interval, pulse rate, minimum frequency, maximum frequency and bandwidth. The minimum frequency, maximum frequency and bandwidth were sampled at three points of the pulse train frequency spectrum, i.e. at the beginning, middle, and end of the pulse.
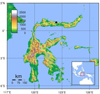 |
Fig. 2 Location of Nantu Wildlife Sanctuary (black star) that lies within the province of Gorontalo, Northern Sulawesi. |
3 Results and Discussion
A total of 31 advertisement calls were recorded for the three males [(Individual A; MZB Amph. 30503; SVL 42.70 mm; 3 calls); individuals B (MZB Amph. 30504; SVL 40.81 mm; 15 calls); individuals C (MZB Amph. 30505; SVL 40.62 mm; 13 calls)]. The tempo of the vocalizations that were produced by L. larvaepartus during the recordings were strongly influenced by the presence of nearby humans, and even a 5-minute recording time for one individual did not result in the male generating his calls following a regular rhythm. Therefore, call rate cannot be determined from this study.
The type of wave from the call of L. larvaepartus is a pulse (Figure 3); with one advertisement call consisting of 1-6 pulses. A total of 31 advertisement calls from three males consisted of 10 advertisement calls with only one pulse (32%), 4 advertisement calls that included two pulses (13%), 6 advertisement calls that included three pulses (19%), 5 advertisement calls that included four pulses (16%), 4 advertisement calls that included five pulses (13%), and 2 advertisement calls composed of six pulses (6%); therefore the number of pulses in one advertisement call varies greatly.
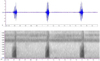 |
Fig. 3 Oscillogram (upper) and spectrogram (lower) of advertisement call of Limnonectes larvaepartus which is formed of three pulse trains. |
3.1 Oscillograms
The sound wave structure of the call of L. larvaepartus (Figure 4) is very different from the sound wave structure of the call of the other Limnonectes that have pulse type calls, including L. dabanus [18] and L. modestus [15]. One call of L. larvaepartus consists of a series of short pulses, with each short pulse formed by one wave period. The interval duration between pulses inside the pulse train was between 1-6 milliseconds (ms) at the beginning to the middle of the pulse train, followed by the short pulse becoming more compressed at the end of the pulse train without interval.
The number of pulse trains in one advertisement call varies greatly; each male can release 1-6 pulse trains in one advertisement call. When there were more pulse trains in an advertisement call, the duration was longer. The duration of an advertisement call that consists of six pulse trains can reach 450 ms, while the duration of a call that is comprised of one pulse train varies considerably; the shortest duration was ~64 ms.
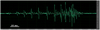 |
Fig. 4 The wave structure of a single pulse train of calls Limnonectes larvaepartus. The bar is equal to 10 ms. |
3.2 Spectogram
The spectrogram of the advertisement call of L. larvaepartus (Figure 5) is also very different from the spectrogram of the calls of other Limnonectes species. Each pulse has a minimum frequency, a maximum frequency and a frequency spectrum of its own frequency which is different from that of another pulse in a pulse train, whereby the pulse attached to the back of the pulse train forms frequency energy with the widest bandwidth. The minimum frequencies decreased from the beginning to the end of the spectrum (see Figure 5, Figure 6 and Table 1), while the maximum frequency and the frequency energy spectrum increased gradually. Of the 31 calls obtained from three males, there was no flat minimum frequency and flat top frequency in a single pulse train. The bandwidth of one pulse train showed that from the beginning to the end of the wave the bandwidth was becoming enlarged at the end (Figure 5 and Table 1). The average bandwidth of the three males showed different frequency values; individual A showed that the bandwidth at three frequency data retrieval points (beginning, middle and end) had the lowest frequency value compared to the other two males. However, the pulse spectrum energy of the L. larvaepartus’s call did not show any dominant frequency; the peaks of the spectrum look almost parallel to each other. The spectral peak sequence will increase as the width of the frequency band increases.
Pulse train rate is quite variable from the three male individuals; the value is between 10.7/sec and 12.7/sec. Each male did not show a different pulse train rate with each other. Call rate cannot be analyzed in these recordings, since the call duration that were produced by the three males were probably influenced by the presence of the person performing the sound recording, so the interval duration among the advertisement calls varied greatly.
Average value, Standard Deviation (SD), range and bandwidth average from three minimum frequency data of retrieval points and three maximum frequency data of retrieval points over one pulse train of three males of Limnonectes larvaepartus.
 |
Fig. 5 One pulse train spectrogram of Limnonectes larvaepartus’s advertisement call. The bar is equal to 10 ms. |
 |
Fig. 6 The average value of the three minimum frequencies from data retrieval points (above) and the three maximum frequencies from data retrieval points (below) for a single pulse train of individual C of Limnonectes larvaepartus. |
3.3 Unusual call characteristic
The sound wave type of the advertisement call of L. larvaepartus is formed by a short pulse sequence, each short pulse producing its own frequency. The oscillogram and spectrogram bandwidth spectrum of L. larvaepartus resemble the sound wave type of the advertisement call of L. hascheanus (Figure 7). The call of L. hascheanus was described by Ohler et.al. [19], but the detailed structure of the sound wave was not clear. The difference between the short pulse of L. larvaepertus and the short pulse of L. hascheanus is in the number of periods that form a pulse; in L. larvaepartus the pulse only consists of one period, whereas in L. haschenus it consists of two periods (Figure 8). The sound wave type of L. hascheanus and L. larvaepartus calls is unusual and very simple when compared with the call sound wave type of L. arathooni [14], L. dabanus [18], L. microdiscus [12], L. palavanensis [13], and members of the Limnonectes group lacking vocal sacs, i.e. L. fujianensis [20], L. hikidai [5], and L. kuhlii [12]. Although the calls of L. larveapartus and L. hascheanus are similar to one another, these species are not closely related [9]; [11] and this similarity therefore might reflect convergent evolution.
 |
Fig. 7 One call oscillogram and spectrogram of Limnonectes hascheanus’s advertisement call. The bar is equal to 20 ms. (Source of call: https://www.ecologyasia.com/verts/amphibians/hill-forest-frog.htm). |
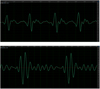 |
Fig. 8 Four short pulses of Limnonectes larvaepartus (above), and two short pulses of L. hascheanus (below). (Source of call of L. hascheanus: https://www.ecologyasia.com/verts/amphibians/hill-forest-frog.htm). |
3.4 Acoustic niche adaptation
The distribution of L. larvaepartus and L. modestus is regionally sympatric [10], but when observed from the microhabitat scale, these two frogs tend to live in different types of habitat. Limnonectes modestus occupies larger rocky river habitats with strong water flow, while the L. larvaepartus typically inhabits narrower and shallower rocky streams and seeps with very slow water current. The spread of the microhabitat scale of these two frogs on the Adudu River was clearly allopatric [15]; L. modestus dominated the fast-flowing Adudu River habitat, while the L. larvaepartus dominated the upstream habitat of Adudu River, the distance the streams of about 100 meters from the Adudu River water body. Iskandar et al [10] hypothesized that L. larvaepartus prefers small streams in order to avoid predation by larger Limonectes species such as L. modestus and L. heinrichi (average body length 70.2 mm), while males of L. larvaepartus are much smaller with an average body size of 37.4 mm (Iskandar et al. 2014; the mean SVL of the three males in this study was 41.38 mm). When considering the frequency spectrum of the advertisement calls of the two frogs, we assume that they should not call at the same time and at the same localities because they have similar acoustic niches; L. modestus has an advertisement call with a bandwidth spectrum of around 2000 Hz [15], however the frequency band of L. larvaepartus is between 1000 Hz to 4800 Hz (Table 1) with a strong energy spectrum that can reach 3600 Hz (Figure 5). This suggests that the frequency band of L. larvaepartus can mask the frequency band of L. modestus. Based on the acoustic niches, these two frogs cannot live together in the same habitat, because the overlap in the acoustic niches disturbs the comfort zone of the other frog acoustic niches, which calls that has wide-frequency band and broad-energy spectrum can interfere the acoustic niches of other frogs species that has calls with a narrow frequency spectrum [21]. Harmonization of acoustics with minimal overlapping of frequency bands is a requirement to occupy the same microhabitat of the frog species group [22].
4 Conclusion
Sound wave structure of call L. larvaepartus is very unique among Limnonectes’s calls that is characterized by row of short pulse that construct pulse train. The advertisement calls also do not have dominant frequency; where as the minimum frequency of one pulse train decreases gradually from the start to the end of pulse train; however maximum frequency increases gradually from the start to the end of pulse train.
We thank the Foreign Research Permit Coordinating Team (TKPIPA) of the Indonesian Ministry of Research, Technology and Higher Education for granting the Research Permit to Dr. Lynn Clayton through Letter of Research Permit No:10/EXT/SIP/FRP/E5/Dit.KI/IV/2017. We are also extremely grateful to the Rainforest Trust, Wildlife Reserves Singapore and People and Plants International for their funding support. We are very grateful to the Indonesian Ministry of Research, Technology and Higher Education for supporting this study under the scheme Counterpart Budget of Foreign Researchers that was awarded to Prof. Dr. Gono Semiadi. We also extend our thanks to BKSDA Gorontalo for the permits and all collaboration in support of research activities. Finally, many thanks are addressed to Dr. Jim McGuire for reviewing and improving the manuscript. Authors contribution: HK and AH contributed equally in this work; LC: coordinating activities in the field and helping in correcting English.
References
- D.R. Frost http://research.amnh.org/herpetology/amphibia/index.htm l [accesed on 1st July 2018] (2018) [Google Scholar]
- M. Matsui, S. Panha, W. Khonsue & N. Kuraishi, Zootaxa 2615, 1–22 (2010) http://dx.doi.org/10.11646/zootaxa.2615.1.1 [Google Scholar]
- M. Matsui, K. Nishikawa & K. Eto, Raffles B Zool 62, 629–687 (2014b) http://zoobank.org/urn:lsid:zoobank.org:pub:9C21B7C4-27AD-4103-89C0-513D2E80106C [Google Scholar]
- D.S. McLeod, S.J. Horner, C. Husted, A.J. Barley, & D.T. Iskandar, Zootaxa 2883, 52–64 (2011) http://dx.doi.org/10.11646/zootaxa.2883.1.4 [Google Scholar]
- M. Matsui & L. Nishikawa, Current Herpetology 33 (2), 135–147 (2014) https://doi.org/10.5358/hsj.33.135 [CrossRef] [Google Scholar]
- M. Matsui, M.D. Belabut & N. Ahmad, Zootaxa 3881, 75–93 (2014a) https://doi.org/10.11646/zootaxa.3881.1.6 [Google Scholar]
- M. Matsui, N. Kuraishi, K. Eto, A. Hamidy, K. Nishikawa, T. Shimada, P. Yambun, C.S. Vairappan, & M.Y.B. Hossman, Mol Phylogenet Evol (2016) https://doi.org/10.1016/j.ympev.2016.06.009 [Google Scholar]
- S.B. Emerson, R.F. Inger, & D. Iskandar, Mol Phylogenet Evol 16, 131–142 (2000) https://doi.org/10.1006/mpev.2000.07788 [Google Scholar]
- B.J. Evans, R.M. Brown, J.A. McGuire, J. Supriatna, N. Andayani, A. Diesmos, D. Iskandar, D.J. Melnick & D.C. Cannatella, Syst Biol 52 (6), 794–819 (2003) https://doi.org/10.1093/sysbio/52.6.794 [PubMed] [Google Scholar]
- D.T. Iskandar, B.J. Evans & J.A. McGuire, PLoS ONE 9 (12), 1–14 (2014) https://doi.org/10.1371/journal.pone.0115884 [Google Scholar]
- M.I. Setiadi, J.A. McGuire, R.M. Brown, M. Zubairi, D.T. Iskandar, N.J. Andayani, J. Supriatna, & B.J. Evans, The American Naturalist 178, 221–240 (2011) https://dx.doi.org/10.5061/dryad.8913. [CrossRef] [PubMed] [Google Scholar]
- H. Kurniati & A. Boonman, Fauna Indonesia 10 (2), 18–27 (2011) https://doi.org/10.5072/RIN/HPNPCG [Google Scholar]
- J.G. Vallejos, T.U. Grafe, H.H.A. Sah & K.D. Wells, Behav Ecol Sociobiol 71–95 (2017) https://doi.org/10.1007/s00265-017-2323-3 [Google Scholar]
- R.M. Brown, & D.T. Iskandar, J Herpetol 34, 404–413 (2000) https://doi.org/10.2307/1565364 [Google Scholar]
- H. Kurniati & A. Hamidy, Jurnal Biologi Indonesia 14 (2), 147–153 (2018) [Google Scholar]
- M.D. Kusrini, J.J.L. Rowley, R. Khairunnisa, G.M. Shea & R.I. Altig, PLoS ONE 10 (1), 1–9 (2015) https://doi.org/10.1371/journal.pone.0116154 [Google Scholar]
- J. Köhler, M. Jansen, A. Rodríguez, P.J.R. Kok, L.F. Toledo, M. Emmrich, F. Glaw, C.F.B. Haddad, M. Rödel & M. Vences, Zootaxa 4251, 001–124 https://doi.org/10.11646/zootaxa.4251.1.1 [Google Scholar]
- J.J.L. Rowley, D.T.T. Le, H.D. Hoang & R. Altig, Zootaxa 3881 (2), 195–200 (2014) https://doi.org/10.11646/zootaxa.3881.2.8 [CrossRef] [PubMed] [Google Scholar]
- A. Ohler, S. Grosjean & JM. Hoyos, Nouv Rev Fr 26: 67-70 (1999) [Google Scholar]
- Y.L. Zhou, X. Qiu, X.B. Fang, L.J. Yang, Y. Zhao, T. Fang, W.H. Zheng & J.S. Liu, Zoological Research 35 (1), 42-50 (2013) https://doi.org/10.11813/j.issn.0254-5853.2014.1.042 [Google Scholar]
- C.I. Medeiros, C. Both, T. Grant & S.M. Hartz, Biol Invasions 19, 675–690 (2017) https://doi.org/10.1007/s10530-016-1327-7 [Google Scholar]
- U. Sinsch, K. Lümkemann, K. Rosar, C. Schwarz & J.M. Dehling, Afr Zool 47 (1), 60–73 (2012) https://doi.org/10.1080/15627020.2012.11407524 [CrossRef] [Google Scholar]
All Tables
Average value, Standard Deviation (SD), range and bandwidth average from three minimum frequency data of retrieval points and three maximum frequency data of retrieval points over one pulse train of three males of Limnonectes larvaepartus.
All Figures
 |
Fig. 1 Adult male of Limnonectes larvaepartus (MZB Amph. 30503) from Nantu Wildlife Sanctuary, Gorontalo, northern Sulawesi (Photograph by A. Hamidy). |
| In the text | |
 |
Fig. 2 Location of Nantu Wildlife Sanctuary (black star) that lies within the province of Gorontalo, Northern Sulawesi. |
| In the text | |
 |
Fig. 3 Oscillogram (upper) and spectrogram (lower) of advertisement call of Limnonectes larvaepartus which is formed of three pulse trains. |
| In the text | |
 |
Fig. 4 The wave structure of a single pulse train of calls Limnonectes larvaepartus. The bar is equal to 10 ms. |
| In the text | |
 |
Fig. 5 One pulse train spectrogram of Limnonectes larvaepartus’s advertisement call. The bar is equal to 10 ms. |
| In the text | |
 |
Fig. 6 The average value of the three minimum frequencies from data retrieval points (above) and the three maximum frequencies from data retrieval points (below) for a single pulse train of individual C of Limnonectes larvaepartus. |
| In the text | |
 |
Fig. 7 One call oscillogram and spectrogram of Limnonectes hascheanus’s advertisement call. The bar is equal to 20 ms. (Source of call: https://www.ecologyasia.com/verts/amphibians/hill-forest-frog.htm). |
| In the text | |
 |
Fig. 8 Four short pulses of Limnonectes larvaepartus (above), and two short pulses of L. hascheanus (below). (Source of call of L. hascheanus: https://www.ecologyasia.com/verts/amphibians/hill-forest-frog.htm). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.



