| Issue |
BIO Web Conf.
Volume 20, 2020
1st International Conference on Tropical Wetland Biodiversity and Conservation (ICWEB 2019)
|
|
|---|---|---|
| Article Number | 03008 | |
| Number of page(s) | 4 | |
| Section | Wetland Plant and Microbes Biodiversity | |
| DOI | https://doi.org/10.1051/bioconf/20202003008 | |
| Published online | 01 June 2020 | |
The Abundance of Arbuscular Mycorrhiza Infective Propagules Under Galam Stand at Shallow Peat of South Kalimantan
1 Banjarbaru Environment and Forestry Research and Development Institute, Banjarbaru, South Kalimatan
2 Faculty of Mathematics and Natural Science, Lambung Mangkurat University, Banjarbaru, South Kalimantan
Colonization of arbuscular mycorrhiza on plants has been reported to give benefit to p lants, especially at extreme sites such as degraded peatland. Galam (Mela leuca ca juputi) is an indigenous peatland species which grows on acidic condition. The number of arbuscular mycorrhiza infective propagules is important to be determined concerning the galam regeneration due to its offered benefits that support colonization. This research aims to determine the abundance of arbuscular mycorrhiza infective propagules under galam stand and to describe symbiotic forms of AMF colonization on the roots of galam. The Most Probable Number (MPN) method, wet sieving, and root staining from the modification of Vierherlig et al., 1996, and the calculation of root’s mycorrhizal colonization by grid line technique were the methods that were used in this research. The research used Complete Randomized Design (CRD) with a 5-fold factorial pattern. The results of this study indicated a significant difference between the abundance of AMF under galam stands at the depth of 0-15 cm and 15-30 cm respectively . The results of spores identification showed 4 genera of spores, namely: Glomus, Gigaspora, Scutellospora, and Acaulospora. The structure of root colonizations were hyphae, spores, vesicles, and arbuscular.
© The Authors, published by EDP Sciences, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Galam (Melaleuca cajuputi) is a woody plant species grows on shallow to moderately deep peat (1-2 m), which can adapt to acidic conditions with the pH range from 3-7. This plant has been recorded on shallow to moderately deep peat (1-2 m) in South Kalimantan [1]. Moreover, Galam can grow under flooded conditions due to the adaptation of its metabolisms [2]. It is called paperbark in English due to its thin and papery white bark [3]. Galam poles are used for construction purposes, sawn timber, high-quality fuelwood, and charcoal. Due to the high demand for timber, the availability of Galam has been decreasing due to overexploitation. Galam is not cultivated. It has relied on natural regeneration and no yield management in the field. Thus, the sustainability of Galam trees is threatened.
Most plants, including Galam, associates with arbuscular mycorrhiza fungi (AMF). AMF has the main three functions including increasing plant growth, enhancing the absorption of soil nutrients, and protecting plants from the pest and disease attack and critical site condition [4]. To conserve Galam, we need to know the benefit of AMF colonization on Galam. One of the ways to determine the benefit of AMF colonization is by providing the number of infective propagules under the Galam trees. Infective propagules including spores and sporocarps, extraradical mycelium, and colonized roots; establish associations when they contact potential host-roots [5]. The objective of this research is to determine the abundance of arbuscular mycorrhiza infective propagules under Galam stand and to describe symbiotic forms of AMF colonization on the roots of Galam.
2 Methods
2.1 Soil and root sample collection
Soil samples and root samples were collected under the galam trees of Liang Anggang shallow peat at South Kalimantan. Root sample collection was carried out unproportionally fro m five galam trees; put in the envelopes and labeled. Soil samples were collected from 5 galam trees and within each tree, samples were replicated from 3 points. The number of soil samples was approximately 1 kg for each point at two different depth: 0-15 cm and 15-30 cm. The soil samples were put in plastic bags and labeled.
2.2 The Most Probable Number (MPN) method
To obtain the number of infective propagules, a series of 4-fold dilutions were prepared. The soil samples of 1 kg were air-dried and sieved at 0.5 cm x 0.5 cm sieve. This sample was used as a base for the next dilution and labeled substrate A. Then, 4 kg of soil sample was autoclave-sterilized, sieved, and dried. This sample is labeled as substrate B. Furthermore, 11 kg of soil was autoclave-sterilized, un-sieved. This sample then labeled as substrate C. The MPN method was then started with putting 100 g of substrate C in each pot. Then, the dilution of substrate A and B was applied 50 g in each pot. The top layer of the pots was put 50 g of substrate C. Three maize seeds were then sown in each pot, watered daily, and grown for 4 weeks. After 4 weeks, the plant’s roots were then harvested, cleaned, and stained with the modification of the Vierheilig method [6]. In this method, the root samples were washed with tap water, soaked them in KOH 10% for 24 hours, soaked in HCl 0.1 N for 24 hours, and ink + vinegar 5% solution for 24 hours. The stained root samples were then checked for mycorrhizal colonization, counted, and reported as infected (+) or not infected (-).
2.3 The wet sieving method for isolating the AMF spores
One hundred grams of soil sample was mixed with 1000 ml of tap water and mixed well. Then, it was poured onto a series of sieve 250, 125, 75, and 63 µm. The particle that was trapped in the sieve was then moved onto a Petri dish, checked, and counted under a stereomicroscope. The spores were then distinguished based on color and size.
2.4 Morphological identification of arbuscular mycorrhiza spores
There were six genera of arbuscular mycorrhiza spores which form a symbiosis with plants namely Glomus, Gigaspora, Scute llospora, Acaulospora, Enthrophospora, and Sclerocystis. The spores were identified based on color, wall layer, Melzer’s reaction, bulbous suspensor, and sustending hyphae [7].
2.5 Data analysis
The infective propagules were counted using a formula
Log Ω = x . log a – K
Ω: the number of infective propagules
X: average number of infected pots
a: dilution factors
K: taken from Sieverding [8].
The number of spores and the arbuscular mycorrhiza colonization in plant’s roots was presented descriptively.
3 Results
3.1 Number of infectives propagules
The number of infective propagules under the Galam stand is presented in Table 1.
The number of infective propagules under the Galam stand varied with the lowest of ± 55667,28 and the highest of ± 85113,8. The average number of arbuscular mycorrhiza infective propagules under the Galam stand was ± 75298,29.
The number of infective propagules under the Galam stand.
3.2 The arbuscular mycorrhiza spores
The arbuscular mycorrhiza spores that were isolated under the Galam trees were varied. There were six species namely Glomus sp. 1, Glomus sp. 2, Glomus sp.3, Gigaspora sp., Acaulospora sp., and Scutellospora sp. The spores were presented in Figure 1.
The average number of spores per 100-gram soil is presented in Table 2.
The average number of spores under galam trees at the soil depth of 0-15 cm and 15-30cm were 508.4 and 337.2, respectively.
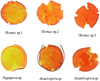 |
Figure 1 Arbuscular mycorrhiza spores under Galam stand at shallow peat of South Kalimantan. |
The average number of spores per 100-gram soil under galam trees.
3.3 Colonization of arbuscular mycorrhiza on roots
The percentage of roots colonization is presented in Table 3.
The arbuscular mycorrh iza colonization on the roots of galam at shallow peat of Kalimantan at 0-15 cm and 15-30 cm depth were 70 % and 57.42%, respectively. Both were categorized as high according to O’Connor [10].
The symbiotic forms of AMF colonization on the Galam roots were found namely arbuscular and vesicular (Figure 2).
The AMF arbuscular structures look like a tree; consists of intraradical hyphae. Arbuscules functions as a place for the exchange of nutrients and carbon between AMF and host plant. The molecular and structural organizations of arbuscular facilitate and regulate the processes of nutrient exchange [9]. The vesicles are terminal swellings of hyphae formed inter and intracellularly having a storage function [7].
Arbuscular mycorrhiza colonization on galam roots at shallow peat of South Kalimantan.
 |
Figure 2 The symbiotic forms of AMF colonization on Galam roots showing arbuscular (left) and vesicular (right). |
4 Discussion
The average number of infective propagules under the galam tree at shallow peat of South Kalimantan was ± 75298,29 per 50-gram soil; or 1505 per gram soil. According to The Indonesian Ministry of Agriculture Decree No. 70/ 2011 on Biofertilizer Standard, the number of arbuscular mycorrhiza propagules shall higher than 50 per gram soil. Moreover, if we are looking at the root colonization at 0-15 cm and 15-30 cm, both exceeded mo re than 50%. According to O’Connor [10], it was categorized as high colonization. Thus, according to The Indonesian Ministry of Agriculture Decree No. 70/ 2011 on Biofertilizer Standard, the minimum percentage of colonization for the biofertilizer is more than 50%. It means, fro m the number of infective propagules and arbuscular mycorrhiza colonization, we can determine that Galam can get the benefit from arbuscular mycorrhiza.
Glomus was the species that most found under the Galam trees at shallow peat of South Kalimantan, Glomus is known to be adaptable in various soil and has the widest habitat [11]. Glomus has a high tolerance to extreme conditions and can form symbiosis [12]. Ramadhani [13] added that Gigaspora is also able to survive in the extreme condition such as acidic or drought. Yuwati et al [14] also found that Glomus sp was dominantly found at deep after burnt peatland at Central Kalimantan. The number of spores was higher at 0-15 cm than 15-30 cm. The deeper the peat soil, the moisture is higher. Moisture could accelerate the spores to germinate [15]. It means, the deeper the soil, the arbuscular mycorrhiza could be in the form of hyphae than spore. Moreover, Delvian [15] stated that high soil moisture content could accelerate the spore to germinate and form colonization on the host plant’s roots. On the contrary, the formulation of new spores will increase the dry condition and roots colonization will be lower. It is a natural response of arbuscular mycorrhiza to keep its existence in nature.
One of the ways to enhance land productivity is by s ilvicultural techniques; one of them is the utilization of biofertilizer. Considering the number of infective propagules, some spores, and colonization rates, the arbuscular mycorrhiza spores under the galam trees at shallow peat of South Kalimantan is promising to be utilized as biofertilizer.
5 Conclusion
The average number of arbuscular mycorrh iza infective propagules under the Galam stand of shallow peat South Kalimantan were 55667-85113 propagules per 50-gram soil. The number of spores at 0-15 cm and 15-30cm soil depth were 508 and 337, respectively. There were four genera of arbuscular mycorrhiza spores found namely Glomus, Gigaspora, Acaulospora, and Scutellospora. Among the four genera, Glomus was dominantly found. The colonization of arbuscular mycorrhiza on the roots was ranging fro m 57 – 70%; categorized as high. These findings showed that Galam was able to get the benefit of AMF colonization and the soil under galam trees was the potential to be developed as biofertilizer.
References
- W. Giesen. Vegetation of the Negara River Basin. In Zieren, M., Permana, T and Giesen, W (eds.). Proceeding of Workshop on Conservation of Sungai Negara Wetlands Barito Basin, Banjarbaru (6-8 March 1989), (1990) [Google Scholar]
- S. Kogawara, T. Yamanoshita, M. Norisada, M. Masumori, and K. Kojima. Photosynthesis and photoassimilate transport during root hypoxia in Melaleuca cajuputi, a flood-tolerant species, and in Eucalyptus camaldulensis, a moderately flood-tolerant species. Tree Physiology 26, 1413-1423, (2006) [CrossRef] [PubMed] [Google Scholar]
- W. Giesen. Case Study: Melaleuca cajuputi (gelam)–a useful species and an option for paludiculture in degraded peatlands. Sustainable Peatlands for People & Climate (SPPC) Project. Wetlands International. p 16, (2015) [Google Scholar]
- S.E. Smith, D.J. Read. Mycorrhizal Symbiosis. 3rd eds. Elsevier. Amsterdam, (2008) [Google Scholar]
- P. Guadarrama, S.C. Arguero, J.A.R. Zapata, S.L.C. Ricalde, and J.A. Sanchez. Propagules of arbuscular mycorrhizal fungi in a secondary dry forest of Oaxaca Mexico. International Journal of Tropical Biology. 56(1); 269-277, (2008) [Google Scholar]
- H. Vierheilig, A.P. Coughlan, U.R.S. Wyss, and Y. Piche. Ink and Vinegar: A simple staining technique for Arbuscular Mycorrhiza l Fungi. Applied and Environmental Microbiology. 64: 5004-5007, (1996) [Google Scholar]
- Brundrett, M.N. Bougherr, B. Dells, T. Grove, N. Malajczuk. Working with mycorrhizas in forestry and agriculture. Bernie Dell, Murdoch University. Murdoch, WA. 374 p. (1996) [Google Scholar]
- E. Sieverding\. Vesicular-arbuscular mycorrhizal management in tropical agrosystems. Deuttsche Gesellschat Technische Zusammenarbeit (GTZ), Eschborn, Germany, (1991) [Google Scholar]
- Z.M. Solaiman, B. Mickan. Use of Mycorrhiza in Sustainable Agriculture and Land Restoration In Solaiman, Z.M., Abbott, L.K., and Varma, A. (eds) 2014. Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration. Springer, Heidelberg, (2014) [CrossRef] [Google Scholar]
- P.J.O. O’Connor, S.E. Smith, and F.A. Smith. Arbuscular Mycorrhizal Associations in The Southern Simpson Desert. Australian Journal of Botany. 49: 493-499, (2001) [Google Scholar]
- Hermawan, H.A. Muin, & R.S. Wulandari. Kelimpahan Fungi Mikoriza Arbuskula (FMA) pada tegakang ekaliptus (Eucalyptus pellita) berdasarkan tingkat kedalaman di lahan gamb ut. Jurnal Hutan Lestari. 3(1): 124-132, (2015) [Google Scholar]
- F. Sianturi, R. Linda, & S. Khotimah. 2005. Kepadatan spora fungi Mikoriza Vesikular Arbuskular pada tiga tingkat kematangan gambut di Kawasan Hutan Lindung Gunung Ambawang Kabupaten Kubu Raya. Jurnal Protobiont. 4 (2): 96-102, (2005) [Google Scholar]
- F. Ramadhani. Pengaruh Pemberian Pupuk Rock Fosfat dan Berbagai Jenis Mikoriza Vesicular Arbuskular terhadap Produksi Tanaman Kedelai (Glycine max. L. Merril) pada Tanah Gambut Ajamu Labuhan Batu. Skripsi Fakultas Kehutanan USU Medan, (2007) [Google Scholar]
- T.W. Yuwati, S.S. Hakim, D. Alimah, B. Hermawan, & A.A. Musthofa. Keanekaragaman Spora Mikoriza Arbuskula di Hutan Rawa Gambut Kalimantan Tengah. In: Diana, Sulistioadi, Y.B., Karyati, Sarminah, S., Widiati, K.Y., Kuspradini, H., Sari, D.R. dan Mulyadi, R. (eds) Prosiding Seminar Nasional Silvikultur ke-IV, Balikpapan, (2017) [Google Scholar]
- Delvian. Peranan Ekologi dan Agronomi Cendawan Mikoriza Arbuskula. Sumatera Utara (ID): USU Press, (2006) [Google Scholar]
All Tables
Arbuscular mycorrhiza colonization on galam roots at shallow peat of South Kalimantan.
All Figures
 |
Figure 1 Arbuscular mycorrhiza spores under Galam stand at shallow peat of South Kalimantan. |
| In the text | |
 |
Figure 2 The symbiotic forms of AMF colonization on Galam roots showing arbuscular (left) and vesicular (right). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.



