| Issue |
BIO Web Conf.
Volume 20, 2020
1st International Conference on Tropical Wetland Biodiversity and Conservation (ICWEB 2019)
|
|
|---|---|---|
| Article Number | 03007 | |
| Number of page(s) | 4 | |
| Section | Wetland Plant and Microbes Biodiversity | |
| DOI | https://doi.org/10.1051/bioconf/20202003007 | |
| Published online | 01 June 2020 | |
The Benefits of Chalcone and Its Derivatives as Antibacterial Agents: A Review
Departemen of Chemistry, Faculty of Mathematics and Natural Science, Lambung Mangkurat University Jl. A. Yani Km.35.8. Banjarbaru, South Kalimantan, Indonesia
* Corresponding author: kmustikasari@ulm.ac.id
Chalcone is a secondary metabolite compound found in plants. Chalcones contain two aryl rings, namely ring A and B which connected to the α,β unsaturated ketones. Chalcone derivatives are synthesized by various substituent groups in both rings, as well as the types of rings. These variations make chalcone and its derivatives, have interesting bioactivity, one of which is antibacterial. This review is considered the chalcone-derived compounds that have antibacterial bioactivity, including methoxy, hydroxy, prenyl, and halogen groups in ring A or B. Besides, there are two forms of these rings as well such as pyrroly l-furany l-chalcones and indoly l-thiopheny l-chalcone. We hope this review is useful for the development of the synthesis of organic compounds and the discovery of new drug design.
© The Authors, published by EDP Sciences, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Chalcone (1,3-diphenyl-2-propen-1-one) (1) is one of the secondary metabolites, belongs to the flavonoid class, found in plants. Structurally chalcone has two benzene rings, namely rings A and B, which both connected by α, β unsaturated ketones. In other words, chalcone derivatives can be synthesized through a condensation reaction of Claisen-Schmidt. The derivatives have many substituent groups, as well as the types of rings. Because of both differences, these derivatives have many interesting biological activities, one of which is antibacterial.
1.1 Chalcone Derivatives with Various Substituents Group in The Ring
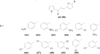
Hasan et al. [1] successfully synthesized chalcone and its derivatives (1-6) and then tested for antibacterial. In their study, these compounds can inhibit the growth of gram-positive and negative bacteria, such as Staphylococcus aureus and Pseudomonas aeruginosa, as well as Escherichia coli and Bacillus cereus, respectively. In this case, the addition of hydroxy and fluoro groups on ring B can increase inhibitory activity against S. aureus and B. cereus. It can be seen from the chalcone inhibition zone (2-3) of both bacteria of 16-35 mm, while the chalcone inhibition zone (1) is only 9-11 mm However, the inhibitory zone value of chalcone (2-3) is greater when compared to the amoxicillin inhibition a positive control (15-25 mm). Further, the inhibitory activity of compounds (2-3) against gram-negative bacteria (E. coli) also shows greater activity when compared to chalcone (1), but is relatively lower than the positive control.
Ocampo et al. [2] succeeded in synthesizing chalcone (1) and its derivatives (7-13) with variations of chloro groups in rings A and B. The compounds showed the antibacterial activity as well against E.coli, P. aeruginosa, and S. aureus. The substitution of the chloro group in the meta and para position of (ring A) in compound (8-9) tends to decrease the inhibitory activity when compared to compound (1). However, the substitution in the ortho position in ring A of compound (7) causing it to become inactive. Interestingly, the substitution of chloro groups in ring B of compounds (10-12) shows the opposite activity, which can increase its inhibitory activity against all three bacteria tested. In this case, the highest inhibitory activity shown by compound (13), approaching the sulfanilamide activity as a positive control.
Synthesis and antibacterial testing of other chalcone derivatives (14-20) have reported by Burmaoglu et al. [3]. The compound tested against S. aureus, E. coli, and P.aeruginosa. In their results, the compound (14) with methoxy group of ring A in the ortho and meta position could inhibit the activity of all bacteria tested, with the MIC values of 31.25, 31.25, and 62.5 µg/mL, respectively. The inhibitory activity of S. aureus is greater than the inhibitory activity of ampicillin as a positive control of the same bacteria. However, when adding one fluoro group in the ortho, meta, and para position in ring B compound (15-17), there was a decrease in the inhibitory activity of all three types of bacteria. Similarly, the addition of two fluoro groups in the ortho position and the ring B meta compound (18), except for S. aureus, which can increase the inhibitory activity until 50%, with the MIC value of 15.6 µg/mL. The decreasing antibacterial activity also very significantly occurs in compounds (19-20) after the methoxy group on ring A is replaced by a hydroxy group.
The antibacterial activity of chalcone (21-23) to Mycobacterium tuberculosis (H37Rv) have studied by Anandam et al. [4]. In their study, the compounds (21) and (22) show the antibacterial activity with MIC90 of 1.6 μg/mL. This value is stronger than the positive control of pyrazinamide (3.125 µg/mL only). The presence of two methoxy groups on ring B (compound 21) does not affect as an antibacterial activity when compared to compound (22), which has only one methoxy group. However, the presence of three methoxy groups in ring B compound (23) can reduce the MIC90 value to 6.25 µg/mL.
The synthesis of prenylated chalcones on ring A was successfully carried out by Marliyana et al. [5], there are differences in the number of prenyl groups in compounds (24-26). All compounds of this study were tested to B. subtilis, S. aureus, E. coli, and P. aeruginosa. Antibacterial test results showed that the compound (24-26) produced the same inhibitory activity against the four types of bacteria, with MIC values of 50, 25, 25, and 25 µg/mL, respectively. The MIC value is relatively much lower when compared to chloramphenicol as a positive control, which ranges between 1.17-12.5 µg/mL. However, the antibacterial activity of the compound (24-26) is still greater than the compound without the prenyl group, for example, compound (14) from the study of Burmaoglu et al. [3]. The MIC values of compounds (14) against S. aureus, E. coli, and P. aeruginosa were 31.25, 31.25, and 62.5 µg/mL.
Vasquez-Martinez et al. [6] succeeded in synthesizing nine polyoxygenated chalcones (27-35), which had a variety of hydroxy and methoxy groups in rings A and B. The compounds were tested for their antibacterial activity against E.coli and S. aureus. However, the MIC value of the nine types of chalcone compounds (27-35) against E.coli is only > 100 µg / mL, which means it is less active in inhibiting the activity of these bacteria. The same thing happened to S.aureus bacteria, except for compound (34) with a MIC value of 50 µg / mL. The compound (34) is substituted by only two hydroxy groups in ring B, with no other substituents in ring A.
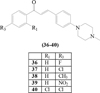
Chalcone derivatives with the addition of piperazinyl groups in the position of para (ring B) have been successfully synthesized and tested for their activity against E.coli, S. typhi, and S. aureus. In this study, the variation of functional groups in ring A, namely the fluoro, chloro, methyl, and nitro groups in the para position. The results showed that the presence of chloro groups in compound (36) generated a greater inhibition to all bacteria tested than nitro (37), fluoro (38), and methyl (39) compound groups. Even the presence of two chloro groups in compound (40) in the ortho and para position produces an inhibitory zone which is almost close to the positive control of penicillin [7].
| No | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | H | H | H | H | H | H | H | H | H | H |
| (2) | H | H | H | H | H | H | H | OH | H | H |
| (3) | H | H | H | H | H | H | H | F | H | H |
| (4) | H | H | H | H | H | H | H | OCH3 | H | H |
| (5) | H | H | H | H | H | H | H | N(CH3) | H | H |
| (6) | H | H | H | H | H | H | H | NO2 | H | H |
| (7) | H | H | H | H | Cl | H | H | H | H | H |
| (8) | H | H | H | Cl | H | H | H | H | H | H |
| (9) | H | H | Cl | H | H | H | H | H | H | H |
| (10) | H | H | H | H | H | Cl | H | H | H | H |
| (11) | H | H | H | H | H | H | Cl | H | H | H |
| (12) | H | H | H | H | H | H | H | Cl | H | H |
| (13) | H | H | H | Cl | H | H | H | Cl | H | H |
| (14) | OCH3 | H | OCH3 | H | OCH3 | H | H | H | H | H |
| (15) | OCH3 | H | OCH3 | H | OCH3 | F | H | H | H | H |
| (16) | OCH3 | H | OCH3 | H | OCH3 | H | F | H | H | H |
| (17) | OCH3 | H | OCH3 | H | OCH3 | H | H | F | H | H |
| (18) | OCH3 | H | OCH3 | H | OCH3 | F | H | H | F | H |
| (19) | OH | H | OH | H | OH | F | H | H | H | H |
| (20) | OH | H | OH | H | OH | F | H | H | F | H |
| (21) | OCH3 | CH3 | OCH3 | CH3 | OH | H | H | OCH3 | H | H |
| (22) | OCH3 | CH3 | OCH3 | CH3 | OH | H | H | OCH3 | OCH3 | H |
| (23) | OCH3 | CH3 | OCH3 | CH3 | OH | H | H | OCH3 | OCH3 | OCH3 |
| (24) | OH | H | OCH3 | H | Oprenyl | H | H | H | H | H |
| (25) | OH | Prenyl | OCH3 | H | Oprenyl | H | H | H | H | H |
| (26) | OH | Prenyl | OCH3 | Prenyl | Oprenyl | H | H | H | H | H |
| (27) | H | OCH3 | OCH3 | OCH3 | H | H | H | OH | OH | H |
| (28) | OCH3 | H | H | OCH3 | H | H | H | OH | OCH3 | H |
| (29) | H | OCH3 | OCH3 | OCH3 | H | H | H | OH | OCH3 | H |
| (30) | H | H | OCH3 | H | OCH3 | H | H | OH | OCH3 | H |
| (31) | OCH3 | H | H | OCH3 | H | H | H | H | H | H |
| (32) | H | OCH3 | OCH3 | H | H | H | H | OH | OCH3 | H |
| (33) | H | H | H | H | H | H | H | OH | OCH3 | H |
| (34) | H | H | H | H | H | H | H | OH | OH | H |
| (35) | H | H | OH | H | OH | H | OCH3 | OCH3 | OCH3 | H |
| R1 | R3 | |
|---|---|---|
| 36 | H | F |
| 37 | H | Cl |
| 38 | H | CH3 |
| 39 | H | NO2 |
| 40 | Cl | Cl |
1.2 Chalcone derivatives with various ring types
One of the chalcone derivatives with variations in ring type is the pyrrolyl-furanyl-chalcones (41-50). The compound consists of pyrrole (ring A) and furan (ring B), with substituents variations in ring B.
The compounds (41-50) above were tested for antibacterial properties against S. aureus, E. faecalis, E.coli, and Klebsiella pneumoniae. Test results showed that the ten compounds were not active as antibacterial, their MIC values ranged from 100-400 µg/mL [8].
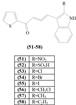
The indolyl-thiophenyl-chalcone (51-58) have successfully synthesized and show antibacterial properties against S. aureus, P. aeruginosa, and E. coli. The compound consists of thiophene (ring A) and indole (ring B). The variations in the nitro, sulfite, methyl, ethyl, halogen, and acyl halide groups were found in ring B. The antibacterial assay showed that almost all variations of the groups, mainly compounds (51-56), generated the inhibitory zones (21-28 mm) close to the positive control of ciprofloxacin. Except for the methyl and ethyl groups in compounds (57-58), their presence can reduce the antibacterial activity, with the inhibition zone of 12-15 mm [9].
| (51) | R=NO2 |
|---|---|
| (52) | R=SO3H |
| (53) | R=Cl |
| (54) | R=Br |
| (55) | R=I |
| (56) | R=CH2Cl |
| (57) | R=CH3 |
| (58) | R=C2H5 |
4 Conclusion
In this review, we discussed the antibacterial properties of some chalcone derivatives. This review is considered the chalcone-derived compounds that have antibacterial bioactivity, including methoxy, hydroxy, prenyl, and halogen groups in ring A or B. Besides, there are two forms of these rings as well such as pyrrolyl-furanyl-chalcones and indolyl-thiophenyl-chalcone. The results of the review indicate that some chalcone derivatives have potential as an antibacterial agent.
References
- S.A. Hasan, A.N Elias, M.S Farhan. Der Pharma Chemica, 7, 2, (2015) [Google Scholar]
- J.A, Ocampo, R. Carrillo, H. Kae, B.O. Ashburn. IJJPR, 13, 3, (2018) [Google Scholar]
- S. Burmaoglu, O.Algul, A. Gobek, A.A. Derya, M. Ulger, B.G. Erturk, E. Kaplan, A. Dogen, G. Aslan, J. Enzyme Inhib Med Chem. 32, 1, (2017) [Google Scholar]
- R. Anadam, S.S. Jadav, A.V. Babu, M.J. Ahsan, H.B. Bollikolla, Med. Chem. Res. 27, 6, (2018) [Google Scholar]
- S.D. Marliyana, D. Mujahidin, Y.M. Syah, Proceeding IOP Conference Series: Materials Science and Engineering, 349, (2018) [Google Scholar]
- Y.A. Martínez, V.M.E. Osorio, D.A.S. Martín, M.A. Carvajal, A.P. Vergara, E. Sanchez, M. Raimondi, S.A. Zacchino, C. Mascayano, C. Torrent, F. Cabezas, S. Mejias, M. Montoya, M.C.S. Martín, J. Braz. Chem. Soc. 30, 2, (2019) [Google Scholar]
- S.S.N. Noorulhaq & M.A. Baseer. AIP Conference Proceedings, 1904, 1, (2017) [Google Scholar]
- A. Özdemir, M.D. Altıntop, B. Sever, H.K. Gençer, H.A, Kapkaç, O, Atlı, M. Baysal M. Molecules, 22, 12, (2017) [Google Scholar]
- VGopi, G., M.D. Dhanaraju., V.G. Sastry. Beni-Suef University. Journal of Basic and Applied Sciences, 5, 3, (2016) [Google Scholar]
All Figures
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.