| Issue |
BIO Web Conf.
Volume 30, 2021
II International Symposium “Innovations in Life Sciences” (ILS 2020)
|
|
|---|---|---|
| Article Number | 02004 | |
| Number of page(s) | 5 | |
| Section | Sorbents – As Factors of Quality of Life and Health | |
| DOI | https://doi.org/10.1051/bioconf/20213002004 | |
| Published online | 22 April 2021 | |
Modification of Zeolites Y and ZSM-5 adsorption of nanoparticles of transition metals from back-micellar solutions for separation of gas mixtures
1
Mendeleev University of Chemical Technology of Russia, Moscow, Russia
2
Institute of Physical Chemistry and Electrochemistry of RAS, Moscow, Russia
* Corresponding author: nerfangorn@gmail.com
On the basis of granular synthetic zeolites NaY, HY, and ZSM-5, adsorbents containing nanoparticles of silver, cobalt, molybdenum, and tungsten were obtained. The samples have a lower surface polarity in comparison with the initial zeolites, which is reflected in the selectivity of a number of samples with respect to argon. This is due to the fact that the argon molecule interacts with zeolites only through nonspecific forces. Modification was performed by interacting with reverse-micellar solutions of nanoparticles. The actual sizes of metal particles and their distribution over the surface of the modified samples of zeolites have been determined by the method of transmission electron microscopy. The samples’ equilibrium adsorption capacities for oxygen and argon (25°С and atmospheric pressure) and the separation coefficient of the argon–oxygen mixture as the ratio of Henry’s coefficients have been determined. It has been demonstrated that samples of the NaY zeolite modified with silver nanoparticles have the separation coefficient value of the argon–oxygen gas mixture equal to 1.6.
© The Authors, published by EDP Sciences, 2021
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Zeolites are widely used in industry for the separation of various gas mixtures, in particular for air separation by the PSA method [1-5]. Modifying the surface of high-silicon zeolites of Y and ZSM types by introducing metal nanoparticles (NPs) is one of the promising directions for creating nanocomposite materials with specified properties for gas purification and separation due to the unique properties of NPs.
The presence of transition metals on the surface of materials changes their adsorption and catalytic properties. Nanocomposites based on γ-Al2O3 with Au or Ru NPs adsorbed on the surface [6] have high catalytic activity in the reaction of hydrogen isotope exchange. An effective ethanol oxidation catalyst was obtained on the basis of Pd and Ag nanotubes deposited on carbon nanotubes [7]. Modification of the silica surface by metal nanoparticles reduces the polarity of these adsorbents. As shown in [8,9], the contributions of specific interactions of polar compounds on silicas containing NPs metals are less than on the initial one. The authors explain this fact by shielding the active centers of the silica gel surface with metal nanoparticles.
Zeolite matrices have a great potential as carriers of transition metals, since they allow to stabilize nanoparticles of certain sizes, in particular metal clusters, without the use of additional stabilizers. In [10-13], the UV spectroscopy method shows high stability of NPs and silver clusters for all the studied types of zeolite matrices, which is expressed in the invariance of the position and intensity of the corresponding absorption bands.
Differences in the surface acidity of the studied zeolite matrices did not affect the stability of the NPs in their structures. The study of catalytic activity of zeolites with different concentrations of the silver NPs [10-13] showed that the optimum content of NPS for carrying out oxidation reactions of H2 and CO is 5-10 wt. %.
Great attention is paid to selection of the synthesis method of NPs metals [14-18], because the method of preparation and the conditions of synthesis of nanoparticles depend on their properties such as size, shape, stability of the particles in time, the possibility of their application to different media, and as a result, adsorption and catalytic properties. In this work were used nanoparticles obtained by synthesizing stable NPs in reverse micelles with characteristic optical absorption spectra and a high ability for adsorption on various substrates [19,20].
At the Frumkin Institute of physical chemistry and electrochemistry of the Russian Academy of Sciences has developed a method for. It belongs to a group of chemical methods in which NPs are obtained by chemical or radiation-chemical reduction of metal ions from their salts to atoms under conditions conducive to the subsequent formation of nanosized metal particles.
NPs deposition on the surface of the carrier can be carried out directly from the reverse micellar solution, that is, the medium of their synthesis, which greatly simplifies the process of obtaining nanocomposites. The small size of metal particles in zeolite, according to the authors [13], is due to nanoscale pores in the zeolite framework, which allow to control the particles size and have a uniform distribution of metal on the internal and external surfaces of the adsorbent. According to IR spectroscopy data, modification of zeolites by transition metal does not lead to a significant change in the degree of crystallinity of high-silicon zeolites [21,22].
The aim of this work was to modify zeolites of types Y and ZSM-5 with transition metal nanoparticles to change the surface polarity of these adsorbents, which was detected by adsorption of air macro-components on the obtained zeolite samples.
2 Experimental
In this work, studies were carried out to modify samples of zeolites of types Y and ZSM-5 by interacting with a suspension of metal nanoparticles (Ag, Co, Mo, W and mixed Mo-W) in produced reverse micellar solution (herein after referred to as RMS).
In the experiment we used granulated zeolites NaY and HY without binding agents and ZSM-5 in H-form with a binder (molar ratio SiO2/Al2O3 ~ 30), provided to us by the Institute of petrochemistry and catalysis of the Russian Academy of Sciences. For research, were used a zeolite fraction with a particle size of 1-2 mm.
Reverse micellar solutions were prepared according to the method given in [8,19,20]. in 0.15 M surfactant solution, which was used as AOT (bis(2-ethylhexyl) sodium sulfosuccinate), the necessary amount of an aqueous solution of 0.3 M AgNO3 (or the corresponding salt) was added in isooctane. The value of the degree of hydration, equal to the molar ratio of water and AOT in the reverse micellar solution, was 5.0. In addition, a polyphenolic compound flavonoid–quercetin (3,5,7,3’, 4’-pentahydroxyflavone) was added as a ion redactant in a 0.15 M solution of AOT/isooctane.
RMS NPs was added to pre-calcined at 350 °C type Y or ZSM-5 zeolite with a particle size of 1-2 mm. The volume ratio of the components zeolite/ RMS of NPs Me/isooctane (medium) was 1:1:1.
The zeolite was kept in solution for several days. The resulting sample was separated on a blue ribbon filter and dried at room temperature.
The content of metal NPs in the solution during interaction with zeolite was controlled using a spectrophotometer SF-2000 (OKB SPEKTR, Russia). For measurement quartz cuvettes with a cross section of 1x1 cm were used. Samples of the solution for analysis were taken 15 min after adding RMS to the zeolite weighed portion, then every 10-15 min, then 30 min, then a day after the start of the experiment, followed by washing with isooctane and ethanol.
The elemental composition of the samples was determined by x-ray fluorescence using the x-MAX INCA ENERGY attachment (Oxford Instruments, UK) to the JEOL JSM-6510 LV electron microscope (JEOL, Japan) at the Mendeleev Center of Collective Use.
The structural and energy characteristics of adsorbents were determined using nitrogen adsorption isotherms at 77 K, taken at the Nova 1200E volumetric unit (Quantachrome, USA). Before measuring the isotherms, the samples were degassed at 400°C and at residual pressure of 10-3 mm Hg for 4 hours. The specific surface area (Ssp) of the samples was calculated using the BET equation, the volume of micropores (W0) and the characteristic adsorption energy (E0) were calculated using the Dubinin – Astakhov equation. The total sorption volume of meso-and micropores (Vs) was determined from the nitrogen adsorption isotherm at a relative pressure value of 0.995.
The microstructure of the obtained samples was analyzed using transmission electron microscopy (TEM) using a LEO 912 AB Omega (“Carl Zeiss”) microscope. The microscope is equipped with an integrated OMEGA energy filter (Zeiss), as well as a digital camera SIS/Olympus Omega 2K. Acceleration voltage of the microscope: 100 kV, image resolution: 0.2-0.34 nm. Sample preparation consisted of grinding the obtained samples in an agate mortar followed by ultrasonic dispersion in ethanol, then the samples were applied to copper grids and dried in air.
Nitrogen, oxygen and argon from cylinders were used as adsorbents. Helium was used as the calibration gas. Nitrogen, oxygen, argon and helium were produced in the Kurchatov Institute and had a purity of: nitrogen − 99.999 vol. % N2; oxygen − 99.999 vol. % O2; helium − 99.995 vol. % He and argon − 99.993 vol.% Ar.
The values of the equilibrium adsorption capacity of samples for nitrogen, oxygen and argon were determined on the basis of kinetic curves of the adsorption of these gases at 25°C and atmospheric pressure, taken on a volumetric unit. The relative measurement error was no more than 5%.
As a measure of the adsorption selectivity of the samples, we used the separation coefficient (Ks), which was calculated as the ratio of Henry’s coefficients at atmospheric pressure and 25°C. It is known that the isotherms of individual adsorption of air macrocomponents on zeolites at room temperature in the range of operating pressures in adsorption oxygen generators are linear, so the adsorption of each of them is considered independent of the adsorption of other components present [23].
3 Results and discussion
The change in the concentration of transition metals in solutions during adsorption and washing with solvents were controlled by the intensity of optical absorption spectra. The optical absorption spectra of RMS silver NPs in presence of NaY zeolite are shown in Fig. 1.
In all experiments, we observed a decrease in the intensity of the optical absorption peak of silver nanoparticles over time, which indicates the transition of NPs metals from the solution to the surface of zeolites.
Fig. 1 also shows that a day after the zeolites were immersed in the reverse micellar solution, the value of optical density (~410 nm) of the absorption band of Ag nanoparticles decreased to a minimum value, which may indicate the adsorption of nanoparticles on zeolites and that the nanoparticles are not washed out with solvents. We observed the similar pictures during the adsorption of NPs of all other studied metals from RMS on NaY, HY and ZSM-5 zeolites
According to the results of elemental analysis, the content of transition metals in the modified samples was low, in the range of 0.3-0.4 wt.%. In table 1, the content of Ag and Co in wt. % is shown in the first column next to the designation of the zeolite sample.
Fig. 2 shows electronic photographs of the Ag / NaY zeolite surface after the interaction of NaY zeolite with NPs Ag RMS. It can be concluded that there is a uniform distribution of particles on the surface of the zeolite with a particle size of 1-3 nm. Figures 3,4 shows the isotherms of nitrogen adsorption at 77 K on the initial zeolites of types Y and ZSM-5, and zeolites modified with metal NPs.
As can be seen from Fig. 3, the nitrogen adsorption isotherms at 77 K on the original NaY zeolite and the Ag/NaY sample are almost identical, for other Mo/NaY, W/NaY and Mo-W/NaY samples, the isotherms are located higher. Nitrogen adsorption on HY in the initial region of the isotherm was significantly higher than on the Ag/HY sample, which indicates that active nitrogen sorption centers were blocked on the modified sample. The structural and energy characteristics of zeolites calculated on the basis of experimental adsorption isotherms are shown in table 1
All samples modified with NPs metals are characterized by a decrease in the values of the characteristic adsorption energy (E0) in comparison with the original zeolites.
For all modified NaY zeolite samples, an increase in Ssp was observed compared to the original NaY. The volumes of micropores (Wo) remained almost unchanged, except for the Mo-W/NaY sample; an increase in Vs values was observed for samples containing tungsten and molybdenum nanoparticles and a mixture of Mo-W nanoparticles. Apparently, metal nanoparticles are located on the outer surface of NaY zeolite particles and in transport pores, which contributed to the creation of an additional volume of sorbing pores and an increasing in the specific surface area.
For HY and ZSM-5 zeolites, there was a slight decrease in Ssp values compared to the original samples. In the case of Ag/HY, there was a decrease in the volume of micropores compared to the original HY while maintaining the Vs value. It follows that the silver nanoparticles were located in the transport pores, while partially blocking the entrance windows to the micropores. A similar conclusion can be drawn about the location of silver and cobalt nanoparticles in samples of zeolites Ag/ZSM-5 and Co/ZSM-5.
The effect of modification of zeolites with metal nanoparticles was investigated by adsorption of air for these samples was 1.2-1.3.
macro-components on the obtained samples. Nitrogen, oxygen, and argon have similar properties. The differences are that nitrogen and oxygen have quadrupole moments, and nitrogen has a quadrupole moment value three times greater than oxygen. Nitrogen and oxygen molecules interact with the surface of adsorbents due to non-specific forces and specific forces (ion-quadrupole interaction). Argon is a molecule with a spherically symmetrical electron shell that interacts with the surface of adsorbents only due to non-specific forces, mainly dispersion attraction.
On the basis of kinetic curves, the equilibrium capacities of adsorbents for nitrogen, oxygen and argon were determined, which are presented in table 2.
According to the table 2, the sample of zeolite Ag/NaY had the highest selectivity to argon. The separation coefficient of the argon-oxygen mixture was 1.6. In this case, the adsorption of nitrogen and oxygen on the Ag/NaY sample was approximately the same, in contrast to the initial NaY, on which nitrogen was adsorbed better. The use of Mo, W nanoparticles and their mixtures as modifiers did not change the nitrogen adsorption on the Mo/NaY, W/NaY and Mo-W/NaY samples in relation to NaY and simultaneously led to an increase in the adsorption of O2 and Ar by 1.5-2 times. For these samples, the selectivity to argon was minimal. The value of the separation coefficient of the argon-oxygen mixture
The HY zeolite was characterized by practically the same adsorption of all gases. After modification with Ag NPs, nitrogen adsorption on Ag/HY decreased by half. Sample Ag/HY had some selectivity with respect to oxygen (the Ks of the O2-N2 mixture was 1.8); for this sample, a slight excess of the adsorption of argon over oxygen was also recorded (the Ks of the Ar-O2 mixture was 1.2).
On ZSM-5 zeolite after modification with Ag and Co nanoparticles observed an increase in the values of the equilibrium capacity for oxygen and argon by about 2 times, while the selectivity to argon was low (Ks = 1.1-1.3).
 |
Fig. 1. Optical absorption Spectra of RMS NPs Ag when adsorbed on NaY zeolite: 1-the initial solution of RMS NCH Ag, 2-the 1st sample, 3-the 2nd sample, 4-the 3rd sample, 5-the 4th sample, 6-a day later, 7-the sample after washing with isooctane, 8-the sample after washing with ethanol. |
 |
Fig. 2. Micrographs of the Ag/NaY sample surface. |
 |
Fig. 3. Isotherms of nitrogen adsorption at 77 K on zeolites NaY and HY: 1 – NaY, 2 – Ag/NaY, 3– Mo/NaY, 4 – W/NaY, 5 – Mo-W/NaY, 6 – HY, 7 – Ag/HY. |
Structural and energy characteristics of Y and ZSM-5 zeolites.
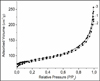 |
Fig. 4. Isotherms of nitrogen adsorption at 77 K on zeolites ZSM-5: 1 – Ag/ZSM-5, 2 – Co/ZSM-5, 3 – ZSM-5. |
Equilibrium capacitirs of zeolites fpr nitrogen, oxygen, and argon and separation coefficient for N2 – O2 and AR – O2 mixtures.
4 Conclusion
By the adsorption of transition metal (Ag, Co, Mo, W and Mo-W mixtures) nanoparticles from reverse micellar solutions on NaY, HY, and ZSM-5 zeolites, samples of modified zeolites were obtained, the adsorption properties of which with respect to air macrocomponents differed from those of the initial adsorbents.
Ag/NaY zeolite with a silver content of 0.4 wt. % had the highest selectivity to argon and the lowest surface polarity among the modified zeolites. Electronic photos of the Ag/NaY zeolite surface after interaction with RMS showed a uniform distribution of Ag NPs (1-3 nm) on the zeolite surface.
We believe that the decrease in the polarity of the NaY zeolite is due to the uniform distribution of silver NPs on the surface of the adsorbent, which screen sodium cations, which are active centers of nitrogen and oxygen sorption.
As shown in works by Golubeva [10-13], differences in the acidity of the surface of zeolite matrices did not effected on the stability of silver NPs in their structures. In zeolites HY and ZSM-5, the centers of nitrogen and oxygen sorption are apparently the protons of hydroxyl groups.
The arrangement of silver nanoparticles on the surface of the HY zeolite and the screening of protons by them led to a 2-fold decrease in nitrogen adsorption and a slight decrease in oxygen adsorption, which are apparently adsorbed on the Ag/HY zeolite due to nonspecific forces. Judging by the equilibrium adsorption values, argon is adsorbed better on Ag/HY zeolite due to its higher molecular weight.
In contrast to NaY and HY zeolites, modification of ZSM-5 zeolite with a binder of silver (and cobalt) nanoparticles led to an increase in oxygen adsorption by 1.7 times. A possible reason for the increase in oxygen adsorption is the presence of a binder (γ-Al2O3). When deposited, metal nanoparticles could appear on both alumina and zeolite.
References
- F.E. Epiepang, X. Yang, J. Li et al., Chem. Eng. Sci., 198, 43, (2019) [Google Scholar]
- X. Yang, F. E. Epiepang, J. B. Li, Y. Wei et al., Chem. Eng. Journal, 362, 482, (2019) [Google Scholar]
- X. Yang, F. E. Epiepang, Y. S. Liu and R. T. Yang, Chem. Eng. Sci., 178, 194, (2018) [Google Scholar]
- Y. R. Wang and R. T. Yang, ACS Sustain. Chem. & Eng., 7, 3301 (2019) [Google Scholar]
- F. Wu, M. D. Argyle, P. A. Dellenback, M. Fan, Progress in Energy and Combustion Science, V.67, 188-205 (2018) [Google Scholar]
- A. A. Odintzov, M. O. Sergeev, A. A. Revina, O. A. Boeva, Uspekhi v himii i himicheskoj tekhnologii, 27, №6, 75–79 (2013) [Google Scholar]
- A.J. Armenta-González, R. Carrera-Cerritos, A. Moreno-Zuria, et al., Fuel, V. 167, 240–247 (2016) [CrossRef] [Google Scholar]
- Belyakova L.D., Larionov O.G., Revina A.A. et al., Protection of Metals, 44, 164-169 (2008) [Google Scholar]
- O. G. Larionov, A.A. Volkov, A. A. Revina, et al., Sorbtsionnye Khromatogr. Protsessy, 5, 713 (2010) [Google Scholar]
- O. Y. Golubeva, N. Y. Ternovaya, N. V. Mal’tseva, D. Meyerstein, Glass Phys Chem, 38, 455–459 (2012) [Google Scholar]
- O. Y. Golubeva, N. Y. Ul’yanova, L. N. Kurilenko, Fiz. Khim. Stekla, 39, 913–919 (2013) [Google Scholar]
- O. Y. Golubeva, N. Y. Ul’yanova, Glass Phys Chem, 41, 537–544 (2015) [Google Scholar]
- N. Y. Ul’yanova, O. Y. Golubeva, Fiz. Khim. Stekla, 5, 479–485 (2018) [Google Scholar]
- J. Talebi, R. Halladj, S. Askari, Journal of materials science, 45, 12, pp. 3318–3324 (2010) [Google Scholar]
- Q.H. Tran, V. Q. Nguyen, A.T. Le, Adv. Nat. Sci: Nanosci. Nanotechnol, V. 4, p. 033001 (2013) [Google Scholar]
- J. García-Barrasa, J. M. López-de-Luzuriaga, M. Monge Central European Journal of Chemistry, 9, 7–19 (2011) [Google Scholar]
- W.C.M Gomes, A.O.W. Neto, P.M. Pimentel, et al. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 426, 18–25 (2013) [Google Scholar]
- D. Singha, N. Barman, K. Sahu, Journal of colloid and interface science, 413, 37–42, 2014. [PubMed] [Google Scholar]
- Revina, A.A., RF Patent 2312741 (2007) [Google Scholar]
- Revina, A.A., RF Patent 2322327 (2008) [Google Scholar]
- S. S. Pavlov, E.S. Astapova, Vestnik amurskogo gosudarstvennogo universiteta. seriya: estestvennye i ekonomicheskie nauki, 49, 39–42 (2010) [Google Scholar]
- E.S. Astapova, V.S. Radomskiy, L.L. Korobicyn and A,V. Filimonov, Nauchno-tekhnicheskie vedomosti SPbGPU. Fiziko-matematicheskie nauki, 49, 54–57 (2010) [Google Scholar]
- Y. I. Shumyatskii, Promyshlennye adsorbtsionnye protsessy (Kolos, Moscow, 2009) [Google Scholar]
All Tables
Equilibrium capacitirs of zeolites fpr nitrogen, oxygen, and argon and separation coefficient for N2 – O2 and AR – O2 mixtures.
All Figures
 |
Fig. 1. Optical absorption Spectra of RMS NPs Ag when adsorbed on NaY zeolite: 1-the initial solution of RMS NCH Ag, 2-the 1st sample, 3-the 2nd sample, 4-the 3rd sample, 5-the 4th sample, 6-a day later, 7-the sample after washing with isooctane, 8-the sample after washing with ethanol. |
| In the text | |
 |
Fig. 2. Micrographs of the Ag/NaY sample surface. |
| In the text | |
 |
Fig. 3. Isotherms of nitrogen adsorption at 77 K on zeolites NaY and HY: 1 – NaY, 2 – Ag/NaY, 3– Mo/NaY, 4 – W/NaY, 5 – Mo-W/NaY, 6 – HY, 7 – Ag/HY. |
| In the text | |
 |
Fig. 4. Isotherms of nitrogen adsorption at 77 K on zeolites ZSM-5: 1 – Ag/ZSM-5, 2 – Co/ZSM-5, 3 – ZSM-5. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.



