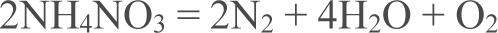| Issue |
BIO Web Conf.
Volume 30, 2021
II International Symposium “Innovations in Life Sciences” (ILS 2020)
|
|
|---|---|---|
| Article Number | 02009 | |
| Number of page(s) | 3 | |
| Section | Sorbents – As Factors of Quality of Life and Health | |
| DOI | https://doi.org/10.1051/bioconf/20213002009 | |
| Published online | 22 April 2021 | |
Simultaneous thermal analysis of mineral fertilizers, purchased in Almaty
Dept. of General and Inorganic chemistry alFarabi Kazakh National University, Almaty, Kazakhstan
* Corresponding author: oksana.ponomarenko@kaznu.kz
Increased demand for agricultural products leads to soil depletion and increased use of mineral and organo-mineral fertilizers. Mineral fertilizers used in agriculture may contain contaminators such as heavy metals or radionuclides that can migrate and accumulate in plants. Although migration and accumulation abilities directly depend on species in which they are presented. Determination of species of heavy metals and radionuclides can be done by sequential extraction technique, which takes long time and a lot of reactants. Preliminary evaluation can be done on the basis of data of simultaneous thermal analysis. In the present study the simultaneous thermal analysis was used for investigation of mineral fertilizers, purchased in Almaty. “Fasko” with ammonia nitrate and “Bujskie udobreniya” fertilizers contain water-soluble fractions and “Ljubo zeleno” and “Fertika” contain organic soluble fractions.
© The Authors, published by EDP Sciences, 2021
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Kazakhstan is a developing country with a growing population and evolving economics [1]. Agriculture plays a significant role in the development of economics of the country. Mentioned economics sector is developing all around Kazakhstan and, especially, in southern regions of Kazakhstan due to better climate conditions and access to water resources. Moreover, South Kazakhstan is the region with biggest population density in the country, two of three megapolises, i.e. Shymkent and Almaty, are situated in the south. Therefore, the tendency of growth of production rates in agricultural sector can be seen annually. Growing food demand leads to soil depletion and in order to solve these problem mineral and organo-mineral fertilizers are widely used. In addition to that, it can be said that soils of South Kazakhstan require regular special treatment with fertilizing agents due to soil types [2].
The biggest issues in application of fertilizers are use of fertilizers above normal concentrations and the contamination of mineral fertilizers with toxicants. Mineral fertilizers may contain radionuclides or heavy metals incorporated in natural apatite, which is the origin of phosphate containing fertilizers, or potassium containing fertilizers may contain K-40, radioactive isotope, above normal concentration. Phosphorus containing fertilizers are more probable to contain uranium and thorium in their content and the concentrations may reach up to 660 mg/kg for U and 220 mg/kg for Th [3]. Species of contaminators is a cause for concern, because it determines migration ability of toxicant and consequently probability of their migration into edible parts of plants [4]. In order to predict the species of contaminators, simultaneous thermal analysis (STA) can be used as fast and simple method.
2 Experimental
Seven samples of mineral fertilizers were purchased in Almaty, namely, “Fasko” granulated superphosphate, “Fertika” organo-mineral fertilizing mixture for vegetables, “Bujskie udobreniya” monopotassium phosphate, “Fasko” fertilizer including ammonia nitrate, “Ljubo zeleno” fertilizer, “Ogorodnik” double superphosphate, “Ogorodnik” phosphate fertilizer. Contents of analyzed fertilizers samples given by producers are presented in Table 1.
Sample preparation included the homogenization of samples with pestle and mortar crushing.
Samples were placed in corundum crucibles and analyzed on NETZSCH STA 449 F3A-0372- M with NETZSCH Proteus software. The identical empty corundum crucible was put with the analyzing sample as a reference. The temperature grew from 30 to 800°C. The heating rate was equal to 20° K/ min. The atmosphere of the analysis was nitrogen gas with purity of 99.99 %.
3 Results and discussion
Seven thermogravimetric differential scanning calorimetry (TG/DSC) curves were obtained as a result of simultaneous thermal analysis. TG/DSC curves are able to characterize the thermal stability of analyzed fertilizers, i.e. they can show decomposition or ignition processes and loss of mass on each step.
On Fig. 1 that corresponds to “Fasko” granulated superphosphate it was seen that decomposition starts at 115° C and at that step absorbed water was evaporated with weight loss of 0.80 %. Gradual mass loss indicates decomposition process on temperatures 130-350 °C. Endothermic process was indicated at temperature range of 440-800 °C with mass loss of 30.31 %. Total mass loss during the analysis was equal to 44.77 %.
In Fig. 2 TG/DSC curve for “Fertika” organo-mineral fertilizing mixture for vegetables is shown, total mass loss in this analysis was equal to 49.14%. Gradual mass loss of 1.88 % at temperatures 90-110 °C indicated evaporation of absorbed water. Exothermic ignition process was presented at range of 200 to 280 °C with massive and severe mass loss of 23.71%, which indicated organic matter ignition. From 290 to 800 °C gradual mass loss was presented with endothermic process at 630-680 °C which indicated decomposition of inorganic components.
“Buiskie udobreniya” monopotassium phosphate TG/DSC curve is shown in Fig. 3. The sample was undergoing endothermic process; total mass change was equal to 12.60 %. Decomposition of sample constituents started at 200 to 340 °C with mass loss of 7.69 %. Weight loss at range from 330 to 380 °C at that step was equal to 4.91 % and corresponded to endothermic process, which was evaporation of crystallised water, further the mass stayed constant with endothermic melting process at 450-470 °C.
“Fasko” fertilizer with ammonia nitrate is presented in Fig. 4. Endothermic process was registered from the very beginning of analysis 40-200 °C which indicated melting processes without mass change. At range of temperature from 275 to 360 °C main mass loss observed which was equal to 97.75 %. It was related to decomposition of main component of the fertilizerammonia nitrate according to the (1). Endothermic process – melting, was registered at 470-480 °C.
Fig. 5 shows TG/DSC curve of “Ljubo zeleno” fertilizer with insignificant mass loss of 1.61 % with respective endothermic effect on differential scanning calorimetry (DSC) in range of temperatures from 60 to 165 °C which identified as dehydration process. Upon further heating up to 300 °C organic matter exothermic process of ignition was seen with mass loss of 17.21 %. From 300 to 800 °C mass loss was equal to 12.79 %, which may indicate decomposition of inorganic matter. Total mass loss was equal to 31.61 %.
In Fig. 6 “Ogorodnik” double superphosphate TG/DSC curve is presented. At 100-110 °C absorbed water evaporated accompanied with 2.59 % mass loss. Heating up to 260 °C led to crystallised water splitting and following loss of 11.79 % of mass. Gradual mass loss was seen up to 700 °C and at temperature range of 700-800 °C severe mass loss was indicated with endo effect. Total mass loss was equal to 34.55 %.
“Ogorodnik” phosphate fertilizer TG/DSC curve is shown in Fig. 7, where multiple decomposition processes were accompanied with gradual mass loss at temperature range of 30 to 390 degrees. From 610 to 800° C staggered endo effect registered with corresponding mass loss. The total mass loss was equal to 20.92 %.
Content of components, reported by producer
 |
Fig. 1. “Fasko” granulated superphosphate TG/DSC curve |
 |
Fig. 2. “Fertika” organo-mineral fertilizing mixture for vegetables TG/DSC curve |
 |
Fig. 3. “Bujskie udobreniya” monopotassium phosphate TG/DSC curve |
 |
Fig. 4. “Fasko” fertilizer with ammonia nitrate TG/DSC curve |
 |
Fig. 5. “Ljubo zeleno” fertilizer TG/DSC curve |
4 Conclusion
High “Fasko” granulated superphosphate, “Ogorodnik” double superphosphate and “Ogorodnik” phosphate fertilizer contains big amounts of water both crystallised and absorbed. On each sample listed above the decomposition processes are registered at temperature ranges from 400 to 800o C.
Five of seven fertilizer samples undergo endothermic processes during the heating, exception is strong exothermic process that observed in “Fertika” organo-mineral fertilizing mixture for vegetables, which indicates large amounts of organic matter in content. Less severe exothermic effect seen in “Ljubo zeleno” fertilizer, which also specify organic substances in content. The organic matter is soluble in organic solvents; thus, migration ability will be high and mobile fraction is organic.
“Bujskie udobreniya” monopotassium phosphate and “Fasko” fertilizer with ammonia nitrate are containing inorganic water-soluble compounds, which allow to predict high potential of migration ability of intoxicants in that water-soluble species.
 |
Fig. 6. “Ogorodnik” double superphosphate TG/DSC curve |
 |
Fig. 7. “Ogorodnik” phosphate fertilizer TG/DSC curve |
References
- Rakhmetova R., Abenova K., Procedia Economics and Finance, 5, 631-636 (2013) [Google Scholar]
- Klebanovich N. V., Efimova I. A., Prokopovich S. ucheb. materialy dlja studentov spec. 1-56 02 02 «Geoinformacionnye sistemy» (2016) [Google Scholar]
- Vogel, C., Hoffmann, M. C., Taube, M. C., Krüger, O., Baran, R., Adam, C., Journal of hazardous materials, 382, 121100 (2020) [PubMed] [Google Scholar]
- Kumar A., Chauhan R. P. “Journal of Radiation Research and Applied Sciences, 7, 454-458 (2014) [Google Scholar]
All Tables
All Figures
 |
Fig. 1. “Fasko” granulated superphosphate TG/DSC curve |
| In the text | |
 |
Fig. 2. “Fertika” organo-mineral fertilizing mixture for vegetables TG/DSC curve |
| In the text | |
 |
Fig. 3. “Bujskie udobreniya” monopotassium phosphate TG/DSC curve |
| In the text | |
 |
Fig. 4. “Fasko” fertilizer with ammonia nitrate TG/DSC curve |
| In the text | |
 |
Fig. 5. “Ljubo zeleno” fertilizer TG/DSC curve |
| In the text | |
 |
Fig. 6. “Ogorodnik” double superphosphate TG/DSC curve |
| In the text | |
 |
Fig. 7. “Ogorodnik” phosphate fertilizer TG/DSC curve |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.