| Issue |
BIO Web Conf.
Volume 17, 2020
International Scientific-Practical Conference “Agriculture and Food Security: Technology, Innovation, Markets, Human Resources” (FIES 2019)
|
|
|---|---|---|
| Article Number | 00061 | |
| Number of page(s) | 5 | |
| DOI | https://doi.org/10.1051/bioconf/20201700061 | |
| Published online | 28 February 2020 | |
Pharmacoprophylaxis of liver diseases: creating a new hepatoprotector
1
Perm Military Institute of National Guard Troops, 614112 Perm, Russia
2
Perm State National Research University, 614990 Perm, Russia
3
Samara state agrarian University, 446442 Kinel, Samara region, Russia
4
Kuban State Agrarian University, 305044 Krasnodar, Russia
5
Moscow Institute of Physics and Technology, 141701 Dolgoprudny, Moscow Region, Russia
* Corresponding author: zykova.sv@rambler.ru
The article presents a study of the hepatoprotective activity of a tricyclic heterocycle, which refers to 5, 6, 7, 8-tetrahydroquinolines. The effect of 8, 8-dimethyl-5-p-tolyl-8, 9-dihydro-2H-pyrido [4, 3, 2-de] cinnolin-3 (7H) was studied on rats under the influence of the model of toxic hepatosis induced by carbon tetrachloride to find out the indicators of peroxidation and biochemical indicators. Biochemical studies have shown that modelling toxic fat hepatosis caused by the inception of carbon tetrachloride to rats increased the activity of alanine aminotransferase by 2.5 times more compared with the intact group, indicating the development of oxidative stress induced by the treatment of pyrido [4, 3, 2] Cinnol I that reduced the toxic effect of CTC by 79.9 %. Mexidol had a less pronounced hepatoprotective effect: the activity of Alanine aminotransferase on animals of the second group was lower by 29.2 % than on rats from the control group. Thus, a new compound with hepatoprotective activity has been developed and studied.
© The Authors, published by EDP Sciences, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
The growth of liver diseases is associated with a number of factors contributing to the growth of metabolic disorders due to the abundance in food of large amounts of saturated fat acids, side effects that occur when taking non-steroidal anti-inflammatory drugs (NSAIDs), anthelmintic and antibacterial drugs. All of these factors directly affect liver. One of the most common non-infectious liver diseases of animals is fat hepatosis. The creation of hepatoprotectors and the expansion of the possibilities of their use are primarily associated with the impact on key units of redox homeostasis. That is why antioxidants play a special role in maintaining normal metabolic processes in the liver, which help to prevent peroxidation processes that may be associated with an excess of free radicals in the body. It is also known that it is lipophilic antioxidants have significant hepatoprotective potential.
2 Materials and methods
2.1 Study subject
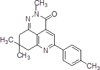
As a drug with hepatoprotective activity, which can be used in the complex therapy and prevention of fat hepatosis, we studied the compound 8,8-dimethyl-5-p-tolyl-8,9-dihydro-2H-pyrido [4,3, 2-de] cinnolyn-3 (7H) – one, having the formula (I):
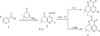
The nearest structural analogues of the claimed compounds are known, which are obtained by the interaction of aroyl pyruvic acid (1) and 3-amino-5,5- dimethylcyclohex-3-enone. As a result, 2-aryl-7,7- dimethyl-5-oxo-5,6,7,8-tetrahydroquinolin-4-carboxylic acids (2) are isolated, which are the starting components for the synthesis of tricyclic heterocycles of 2-substituted 5-aryl-3,7,8,9-tetrahydro-2H-pyrido [4,3,2-de] cinnol-3- ones (3) and 5-aryl-8,8-dimethyl-3,7,8,9-tetrahydro [1,2] oxazino [5,4,3-de] quinolin-3-ones (4) according to the following scheme [1]:
Synthesis of precursors of the compounds of 2- substituted 5-aryl-3,7,8,9-tetrahydro-2H-pyrido [4,3,2- de] cinnolin-3-s (4), which include compound I, the synthesis of which to be carried out earlier [1].
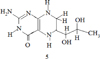
The example of antioxidant activity is 5,6,7,8- tetrahydrobiopterin (BH 4) and its derivatives (5), which showed high antioxidant activity relative to the superoxide anion – a radical generated by paraquat in a cell culture of hepatocytes [2].
The compound (5) is soluble in water, which contributes to insufficient absorption from the gastrointestinal tract, rapid elimination from the body, since lipophilic compounds are used for greater effectiveness of hepatoprotectors: for example, a complex of Silybin bioflavonoid derived from milk thistle and phosphatidylcholine. They form a lipophilic complex, which significantly increases the bioavailability of silybin and thus provides powerful protection of the liver. High hepatoprotective activity is observed in a number of heterocyclic compounds [3, 4].
The example of the hepatoprotective effect of heterocycles is polycyclic adamantane derivatives [5].
It is important for the hepatoprotectors to have less toxicity, since the liver with developing hepatosis bears the main detoxifying function of the majority of xenobiotics entering the body.
2.2 Biological method
Hepatoprotective effect was studied on male rats weighing 180-220g on a model of chemically induced hepatosis in vivo, caused by the introduction of carbon tetrachloride (CTC). CTC is administered orally at a dose of 0.4 ml / 100 g of animal body weight. All animals participating in the experiment were divided into four groups: intact group, control (with carbon tetrachloride) and two experimental ones. After 14 days of experience, animals under fluorothane anesthesia of all groups were subjected to decapitation slaughter, and conducted pathological toxicity studies. The animals of all groups were kept on the same diet for pathological examination.
3 Results
Biochemical studies have shown that when modelling toxic fatty hepatosis caused by administering carbon tetrachloride (CTC) to rats (control group), the activity of alanine aminotransferase increased 2.5 times compared with the intact group. At the same time, treatment of animals with pyrido [4,3,2-de] cinnolin I reduced the toxic effect of CTC by 79.9%. Mexidol had a less pronounced hepatoprotective effect: the activity of Alanine aminotransferase in animals of the second group was lower by 29.2% than in rats from the control group. The data on changes in the activity of alanine aminotransferase under the action of the studied compounds indicate its marked hepatoprotective effect.
The activity of aspartate aminotransferase on rats with toxic fat hepatosis significantly increased the number of Aspartate aminotransferase in the control group was 2.8 times higher compared with the intact group. At the same time, the increase in the activity of Aspartate aminotransferase in the 1st experimental group treated with the studied compound was expressed to a much lesser extent in comparison with the control group. In turn, the treatment of rats with Mexidol had a lower protective effect, leading to a decrease in the activity of Aspartate aminotransferase by 55.5% compared with the control.
The value of alkaline phosphatase activity on animals with toxic fat hepatosis with the introduction of CTC increased by 18.5%. At the same time, in both experimental groups, the enzyme activity was close to that in animals of the intact group.
One of the important indicators of the overall functional activity of the liver is the level of bilirubin. When modelling the toxic fat hepatosis caused by the inception of CTC, an increase in the level of bilirubin was observed in the control group relative to intact animals by 4.9 times. In the 1st experimental group, the level of bilirubin has increased by 2.4 times, and in the treatment with Mexidol, by 3 times, which indicates the protective effect of the compounds under study.
In the control group (rats receiving CTC without treatment) compared to the intact group, lipid peroxidation products were exceeded: diene conjugates – by 47.4%, malonic dialdehyde – by 84.6%, which indicates the development of oxidative stress induced by CTC. The activity of catalase and superoxide dismutase decreased by 49.8% and 20.6% respectively.
In experimental group 1 and 2, where animals were treated with pyrido [4,3,2] cinnolin or mexidol, respectively, compared to control, the level of DC and MDA was significantly lower and practically did not differ from the corresponding values in the intact group. The treatment of the studied compound of animals from the first experimental group completely prevented the decrease in catalase activity, while the SOD level was slightly lower (by 7.5%) than in the intact group. Despite the pronounced antioxidant activity of Mexidol, the level of catalase and SOD in the second experimental group was lower than in the first experimental group (Table 1).
The accumulated experience of work of law enforcement agencies with service dogs indicates that the successful solution of the problems of using dogs as biodetectors depends on the physiological state, working qualities directly related to the breeding, maintenance and saving of animals. At the same time, one of the most important elements of keeping service dogs is their feeding and physical training. During the work with dogs with impaired metabolism, there is a worsening or complete loss of appetite, a decrease in body weight, depression and lethargy, loss of working ability, an increase or decrease in body temperature, pulse rate and respiration. Possible violation of the digestive tract and the appearance of cough and abnormal nasal secretions are seen. Shortness of breath is observed, the work of the heart, as a rule, is impaired. In addition service dogs of both genders do not show sexual activity.
The symptoms of hypotrophy-deficiency of body weight of varying degrees, caused by a lack of nutrition, and hypertrophy — an increase in the volume and mass of organs, tissue, and cells – are distinguished.
Often, metabolic syndrome can be a signal of the development of liver diseases such as hepatitis, hepatosis (adipose, amyloid), cirrhosis, fascioliasis, echinococcosis, opisthorchiasis, cystomatosis, tuberculosis and some others, considered as hepatic syndromes. We must also not to rule out diseases of the biliary tract: cholecystitis, cholangitis and gallstones.
A special role in the prevention of diseases of the gastrointestinal tract is the use of probiotics that have radioprotection [8]. Liver is the central organ of the homeostasis of the body, metabolic processes play a direct role in providing the body’s energy needs with glucose and converting various energy sources (free fat acids, amino acids, glycerin, lactic acid, etc.) into glucose (the so-called gluconeogenesis), replenishing and storing quickly mobilized energy reserves in the form of glycogen and the regulation of carbohydrate metabolism, replenishment and storage of certain vitamins (especially there is a large amount of the reserves of liposoluble vitamins A, D, water-soluble vitamin B12 in the liver), as well as cations and a number of trace elements, in particular, iron, copper and cobalt cations, is involved in the metabolism of vitamins A, B, C, D, E, K, PP and folic acid, synthesis cholesterol and its esters, lipids and phospholipids, lipoproteins and the regulation of lipid metabolism, the synthesis of bile acids and bilirubin, the production and secretion of bile [9].
Liver damage and the occurrence of syndromes may also be associated with unbalanced feeding of the animal. Care must be taken about the quality of the dog’s food.
Pectin substances [10] have a special role. We have studied the effect of the extract of Salsola Pall on the biochemical parameters in dogs. Salsola Pall is a weedy, annual, spring later plant of spherical shape. The height of the bush is up to 100 cm. It blossoms from July to September. Fruiting is in September and later. It grows in forest-steppe and steppe zones. It weeds litter crops and tilled crops. It is resistant to arid climate and salt-enriched soils.
Salsola Pall is an alternative source of biologically active substances and helps to improve the functional state of the liver. It is distributed in the Middle Volga and Trans-Volga regions, the south of the West Siberian region, the Far East, in the high-altitude valleys of the southern regions of Central Asia. This plant is a powerful hepatoprotector, which also has immunomodulatory, antioxidant, choleretic, lipotropic, and hypoglycemic properties. Salsola Pall (Latin Salsola collina Pall.) belongs to the genus of herbaceous and shrubby plants Salsola (Salsola), belonging to the Amaranth family (Latin Amaranthaceae). Numerous experiments have proved the ability of Salsola Pall to act as a hepatoprotector, that is, to contribute to the normalization of the functions, structure and metabolism of the liver parenchyma. Preparations of halophytic colus are prescribed for liver cirrhosis, cholecystitis, hepatosis, acute, viral and toxic hepatitis [11, 12]. They are used to protect the liver during the treatment of various diseases with drugs with a hepatotoxic effect, with helminthiases and infectious processes in the liver [13, 14]. They have an insulin-like effect; it means that they are able to reduce the sugar content in the blood. As a dietary supplement, Salsola Pall helps to strengthen bones, has an immunostimulating effect, and serves to prevent disorders of lipid metabolism and cholesterol metabolism.
Table 2 shows the biochemical parameters in the application of the extract of Salsola Pall in two groups – experimental and control, consisting of ten dogs each.
Biochemical parameters of blood plasma under the action of compound I
Results of application of the extract of Salsola Pall
4 Conclusion
Thus, the obtained results indicate that compound I possesses significant hepatoprotective activity, exceeding the action of the antioxidant – Mexidol.
As the study of pharmaco-toxicological properties of the heterocyclic pyrido [4,3,2-de] cinnoline parameters were analyzed determining the level of lipid peroxidation (LPO) (malondialdehyde (MDA), diene conjugates (DC)) and the activity of key antioxidant enzymes (superoxide dismutase (SOD), catalase) [7].
In the control group (rats treated with carbon tetrachloride (CTC) without treatment), there was an increase in the level compared to the level in the intact group of LPO products (DC – by 47.44%, MDA – by 84.62%), which indicates the development of oxidative stress induced by CTC. It was found that in the control group the level of SOD activity was reduced by 20.64% (p<0.05) and catalase (p<0.05) decreased by 50.21%.
The level of DC in the group treated with pyrido [4,3,2-de] cinnolin, in comparison with the level in the intact group, is higher by 11.10%, and MDA – by 10.26% (p<0.05). At the same time, the level of SOD enzyme activity is 7.53% (p<0.05) lower than the level of enzyme activity in the intact group, and catalase is 1.17% lower than the level of catalase activity in the intact group (p<0.05). According to the results of pathological studies, the livers of a group of animals after treatment with pyridoxinoline contained insignificant deviations, indicating the development of initial changes in fat hepatosis.
It is possible to use the compound 8,8-dimethyl-5-p-tolyl-8,9-dihydro-2H-pyrido [4,3,2-de] cinnolin-3 (7H) (I) in veterinary medicine to reduce peroxidation of lipid cell membranes with endothelial dysfunction, increase resistance to hypoxia and to prevent the development of fat hepatosis.
Hepatoprotective activity of the compound 8,8- dimethyl-5-p-tolyl-3,4,7,8-tetrahydro-2H-pyrido [4,3,2- de] cinnolin-3 was proven in vivo on a model of acute toxic hepatosis induced by carbon tetrachloride. It is established that this compound affects the indicators that determine the level of free-radical oxidation (FRO): malonic dialdehyde (MDA) and diene conjugates (DC), as well as the activity of the main antioxidant enzymes – superoxide dismutase (SOD) and catalase. Hepatoprotective activity was analyzed by the level of activity of the enzymes – alanine aminotransferase (AlAT) and aspartate aminotransferase (AsAT), alkaline phosphatase, as well as the level of bilirubin.
We also studied the hepatoprotective activity of the extract from the seeds of amaranth and the extract of hilum on a significant sample of dogs. The results of blood biochemical parameters, which indicate the normalization of the level of activity of liver enzymes (alanine aminotransferase and aspartate aminotransferase), were obtained, and positive changes in the lipid profile were noted.
After the application of the extract of Salsola Pall, it was found that the level of the key enzyme – alanine aminotransferase in the experimental group decreased by 22.4%, cholesterol – by 21.2%, alkaline phosphatase – by 24.8%.
The use of the extract allowed restoring the glycogen-synthesizing role of the liver, which led to an increase in glucose level by 15.2%.
Thus, the use of both a synthetic component and an extract of Salsola Pall give a decrease in the activity of the enzymes -hepato indicators – Alanine Aminotransferase and Aspartate Aminotransferase. The mechanism of hepatoprotection consists of the restoration of the radical-binding functions of the liver and the cytoprotective properties [15].
It is possible to use the compound 8,8-dimethyl-5-p-tolyl-8,9-dihydro-2H-pyrido [4,3,2-de] cinnolin-3 (7H) (I) in veterinary medicine to reduce peroxidation lipid cell membranes with endothelial dysfunction, increase resistance to hypoxia and prevent the development of fatty hepatosis.
Hepatoprotective activity of the compound 8,8- dimethyl-5-p-tolyl-3,4,7,8-tetrahydro-2H-pyrido [4,3,2- de] cinnolin-3 was proven in vivo on a model of acute toxic hepatosis induced by carbon tetrachloride. It is established that this compound affects the indicators that determine the level of free-radical oxidation (FRO): malonic dialdehyde (MDA) and diene conjugates (DC), as well as the activity of key antioxidant enzymes – superoxide dismutase (SOD) and catalase. Hepatoprotective activity was analyzed by the level of activity of the enzymes – alanine aminotransferase (AlAT) and aspartate aminotransferase (AsAT), alkaline phosphatase, as well as the level of bilirubin.
We also studied the hepatoprotective activity of the extract from the seeds of amaranth and the extract of Salsola Pall on a significant sample of dogs. The results of blood biochemical parameters, which indicate the normalization of the level of activity of liver enzymes (alanine aminotransferase and aspartate aminotransferase), were obtained, and positive changes in the lipid profile were noted.
The studies of individual components of drugs of hepatoprotective action have shown that the active ingredient is 8,8-dimethyl-5-p-tolyl-3,4,7,8-tetrahydro- 2H-pyrido [4,3,2-de] cinnolin-3 has a pronounced anti-toxic effect that can stop the process of hepatocyte cytolysis. The combination of this substance with the extract of Salsola Pall can expand the possibilities of using the drug in order to prevent the development of hepatosis. Such combination of synthetic and herbal components is an example of the expansion of the mechanisms of the hepatoprotective action and allows the use of this drug as means of therapy, and as a preventive hepatoprotective agent.
References
- D.A. Rudenko, S. N. Shurov, M. I. Kodess et al., Synthesis of 2-substituted 7, 7-dimethyl-5-oxo-5, 6, 7, 8-tetrahydroquinoline-4-carboxylic acids, Organic Chem. J., 48(6), 803–807 (2012) [Google Scholar]
- S. Kojima, S. Ona, I. Lizuka et al., Antioxidant Activity 5, 6, 7, 8-Tetrahydrobiopterin and Its Inhibitory Effect on Paraquat-Induced Cell Toxicy Incultured Rat Hepatocytes, Free Radicales, 23(5), 419–430 (1995) [CrossRef] [Google Scholar]
- S.V. Chepur, V.N. Bykov, M.A. Yudin et al., Features of experimental modeling of somatic and neurological diseases to assess the effectiveness of drugs, Biomedicine, 1, 16–18 (2012) [Google Scholar]
- A.B. Vyshtakalyuk, N.G. Nazarov, V.V. Zobov et al., Evaluation of the Hepatoprotective Effect of L-Ascorbate 1-(2-Hydroxyethyl)-4, 6-Dimethyl-1, 2-Dihydropyrimidine-2-One Upon Exposure to Carbon Tetrachloride, Bull. Exp. Biol. Med., 162(3), 340–342 (January 2017) DOI: 10.1007/s10517-017-36108 Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/28091907 [CrossRef] [PubMed] [Google Scholar]
- L.A. Piruzian, A.S. Kabankin, L.I. Gabrielyan, N.V. Ostapchuk, Hepatoprotective effect of adamantane derivatives and analysis of the connection structure detoxifying activity, J. of Chem. and Pharm., 38(3), 19–25 (2004) [CrossRef] [Google Scholar]
- A. Melhem, M. Stem, O. Shibolet et al., Treatment of chronic hepatitis C virus infection via antioxidants. Results of a phase clinical trial, J. CIin. Gastroenterol, 39, 737–742 (2005) [CrossRef] [Google Scholar]
- D.A. Rudenko, T.V. Shavrina, S.N. Shurov, S.S. Zykova, Synthesis and antioxidant activity of tricyclic compounds containing a 5, 6, 7, 8tetrahydroquinoline moiety, Pharm. Chem. J., 48(2), 100–103 (2014) [Google Scholar]
- K.V. Sychev, E.S. Koshpaeva, R.N. Nizamov et al., Gamma-irradiated bifidobacteria establish a protective effect on mice to experimental radiation exposure, BioNanoSci., 8(1), 323–328 (2018) [Google Scholar]
- A.I. Vengerovsky, A.N. Melentyeva, V.N. Burkova, Hepatoprotective and antioxidant effects of extract of Salsola Pall with paracetamol hepatitis in rats, J. of Chem. and Pharm., B.44, 3, 29–31 (2010) [Google Scholar]
- I.V. Sobol, L.V. Donchenko, L.Y. Rodionova, A.G. Koshchaev, A.V. Stepovoy, Peculiarities of analytical characteristics of pectins extracted from sunflower hearts, Asian J. of Pharm., 11(1), S97–S100 (2017) [Google Scholar]
- S.B. Nikiforov, A.A. Semionov, A.I. Syrchina, Effect of water extract Salsola collina Pall on the distribution of cholesterol between the lipoprotein fractions of rabbit serum in experimental cholelithiasis, J. of Chem. and Pharm., B.34, 10, 27–28 (2002) [Google Scholar]
- Y. Yue, Q. Lin, M. Irfan, Q. Chen, X. Zhao, Characteristics and potential values of bio-oil, syngas and biochar derived from Salsola collina Pall. in a fixed bed slow pyrolysis system, Bioresour Technol, 220, 378–383 (November 2016) DOI: 10.1016/j.biortech.2016.08.028. [Google Scholar]
- M.P. Semenenko, N.N. Zabachta, N.N. Sokolov, E.V. Kuzminova, A study of the pharmacodynamics effects of a complex hepatoprotector on broiler chickens, J. of Pharm. Sci. and Res., 10(1), 146–147 (2018) [Google Scholar]
- M.P. Semenenko, E.V. Kuzminova, F.D. Onishchuk, E.V. Tyapkina, Etiopathogenesis and features of hepatotropic therapy of cows in hepatosis, Veterinary Med., 4, 42 (2016) [Google Scholar]
- G. Wardani, N. Farida, R. Andayani, M. Kuntoro, S.A. Sudjarwo, The Potency of Red Seaweed (Eucheuma cottonii) Extracts as Hepatoprotector on Lead Acetate induced Hepatotoxicity in Mice, Pharmacognosy Res., 9(3), 282–286 (July September 2017) DOI: 10.4103/pr.pr_69_16 [PubMed] [Google Scholar]
All Tables
All Figures
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.