| Issue |
BIO Web Conf.
Volume 9, 2017
40th World Congress of Vine and Wine
|
|
|---|---|---|
| Article Number | 02028 | |
| Number of page(s) | 5 | |
| Section | Oenology | |
| DOI | https://doi.org/10.1051/bioconf/20170902028 | |
| Published online | 04 July 2017 | |
Methanol in wine
1
FIVS, 18 rue d'Aguesseau, 75008
Paris, France
2
Australian Wine Research Institute, Hartley Grove, Urrbrae SA 5064, Australia
This paper examines the origins of methanol in grape wine and the quantities typically found in it, as well as in other foods such as unpasteurised fruit juices. The toxicology of methanol and the associated regulatory limits established by competent authorities in various parts of the world are also considered. It is concluded that such limits are not driven by public health considerations and thus authorities are requested to consider the need for methanol analyses to be performed and reported on certificates of analysis as a condition of market entry for wine. Where methanol limits are still deemed to be necessary to achieve policy objectives, authorities are encouraged to establish them in the light of the levels of methanol typically found in grape wines produced by the full array of internationally permitted winemaking practices, and to consider harmonising their limits with those that have already been established by other governments or recommended by appropriate intergovernmental organisations.
© The Authors, published by EDP Sciences 2017
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0 (http://creativecommons.org/licenses/by/4.0/).
This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0 (http://creativecommons.org/licenses/by/4.0/).
1. Introduction
The origin and significance of methanol in wine, and the associated establishment of regulatory limits for its presence there, are causes of much confusion and misunderstanding in international trade. This paper, produced by the FIVS Scientific and Technical Committee, reviews the topic in some detail, providing reference materials to assist with further study. It concludes that the levels of methanol commonly found in grape wines are broadly similar to those that may be found in many freshly squeezed and unpasteurised fruit juices if they are stored for a period of time after squeezing. It is further demonstrated, from a comparison of regulatory limits for methanol in wine with food safety risk assessments that have been conducted by reputable bodies, that the limits themselves do not serve any real food safety purpose. This is because many litres of wine per day or even per hour would need to be consumed (even if the product contained the highest content of methanol permissible in regulations) to even approach intake levels of any known toxicological concern [1].
2. Chemical properties and other information for methanol
Methanol is chemically characterized as follows [2]:
2.1. Chemical Formula, Synonyms, CAS Registry number
-
Chemical formula: CH3OH
-
Synonyms: Methyl alcohol, Carbinol, Wood alcohol
-
CAS Registry Number: 67-56-1.
2.2. Physico-chemical properties
-
Physical appearance: Methanol is a colourless liquid with characteristic odour.
-
Melting Point: –98 °C
-
Boiling Point: 65 °C
-
Solubility in water: Miscible.
3. Origin of Methanol in wine
3.1. Action of pectinase enzymes
3.1.1. Action of endogenous pectinase enzymes on pectin in grapes
Methanol is produced before and during alcoholic fermentation from the hydrolysis of pectins by pectinase enzymes (such as pectin methylesterase) which are naturally present in the fruit. More methanol is produced when must is fermented on grape skins; hence there is generally more in red wines than in rosé or white wines (see Sect. 4 below).
3.1.2. Use of exogenous pectinase enzymes in winemaking
Exogenous pectinase enzymes are permitted for use in winemaking (generally as clarifying agents) in at least the following countries: Australia, Canada, the 28 Member States of the European Union, Japan, the Republic of Georgia, New Zealand, South Africa, and the United States. Their use is also deemed to be an acceptable winemaking practice by the International Organisation for Vine and Wine (OIV) [3]. As with the activity of pectinases naturally present in grapes, the use of exogenous pectinases as a winemaking practice will have the effect of increasing the levels of methanol in the resulting wine.
3.2. Treatment of wine with Dimethyldicarbonate
Dimethyldicarbonate (DMDC) is an effective pre-bottling sterilant, accepted for use in winemaking in Argentina, Australia, Cambodia, Canada, Chile, the 28 Member States of the European Union, the Republic of Georgia, Hong Kong China, Myanmar, New Zealand, Russia, Singapore, South Africa, Thailand, Turkey and the United States, whose use is generally limited in international regulations and recommendations to a maximum treatment of 200 mg/L of wine [4]. For other alcoholic beverages and mixtures of alcoholic and other beverages with an alcoholic strength by volume of less than 15%, the limit on usage is often set at 250 mg/L. The use of DMDC can be important in stabilizing lower alcohol products from additional fermentation in the bottle, and also allows a reduction in the quantity of sulphur dioxide that is used where the oxygen in wine is kept below 1 mg/L. DMDC breaks down rapidly in wine, producing carbon dioxide and leaving methanol at very low levels not harmful to health and other innocuous products in the wine. Methanol at a level of about 100 mg/L is created in wine from a DMDC treatment at the typical maximum treatment level of 200 mg/L [5].
4. Typical levels of methanol in wine
It was noted above that the presence of low levels of methanol in wine is expected due to the action of pectinase enzymes that are naturally present in the grapes. A study of the literature indicates the following information concerning the typical levels of methanol that may be found in wine (these levels generally do not account for any additional amount that may result from a DMDC treatment):
-
Red wines will tend to contain more methanol (between 120 and 250 mg/L of the total wine volume) than white wines (between 40 and 120 mg/L of the total wine volume), because of the longer exposure to grape skins during the fermentation [6].
-
Wines made from grapes that have been exposed to Botrytis cinerea (e.g. late harvest wines, such as Sauternes or Tokay) also have higher methanol levels than standard grape wines (as much as 364 mg/L of the total wine volume) [7].
-
Wines made from non Vitis vinifera grapes tend to contain more methanol than wine from pure Vitis vinifera [8].
4.1. Case study: Typical levels of methanol in Australian wine
A recent survey looked at 150 wines from across Australia to determine typical levels of methanol in commercial wine [9]. The sample set consisted of 90 red and 60 white wines from multiple varieties and vintages. All wines were analysed using a GC-FID in the Australian Wine Research Institute’s ISO 17025 accredited laboratory. No evidence of DMDC treatment (a source of methanol) was found for any of the wines tested.
Typical ranges for methanol found in Australian wines were; 60–280 mg/L in reds (mean 170 mg/L) and 40–120 mg/L in whites (mean 58 mg/L). All wines tested had some methanol content. The main driver for higher methanol levels appeared to be skin contact during processing. Variety or vintage had no significant impact.
4.1.1. Typical values
Results for red and white wines were significantly different. Red wines typically contained higher levels of methanol across a larger range of content, reflecting greater variation in skin contact times. All wines were found to be within Australian and OIV guidelines (Fig. 1).
4.1.2. Impact of variety
No significant differences of methanol content were found based on grape variety. The only difference found was between red and white wines, reflecting the differences in processing for the different wine styles (Fig. 2).
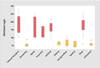 |
Figure 2. Comparison of MeOH concentrations in varietal wines |
4.1.3. Impact of vintage
No significant impact of year of production on the methanol concentration was found (Fig. 3).
 |
Figure 3. Comparison of MeOH concentrations in vintage wines. |
5. Typical levels of methanol in fruit juices
In comparison with the quantities found in wine, non-alcoholic fruit juices naturally contain an average of 140 mg/L of methanol, in a range from 12 mg/L to as much as 640 mg/L. This is quite similar to the average methanol level in white wine and within the range of values seen for red wines [10–12].
6. Toxicology of methanol
Methanol is a toxic chemical. Acute methanol poisoning symptoms resemble those of ordinary alcoholic intoxication followed by the presence of severe upper abdominal pain, visual disturbance sometimes proceeding to incurable blindness and prolonged coma, which may terminate in death from respiratory failure. The fatal dose varies but is usually from 100 mL to 200 mL of methanol. Permanent blindness has been claimed to have been caused by as little as 10 mL. The toxicological properties of methanol are mediated by those of ethanol, and patients suffering from methanol poisoning may sometimes be treated by administration of ethanol [13, 14].
According to a paper produced by the United Nations World Health Organization International Programme on Chemical Safety, it is difficult to perform a risk assessment on methanol because the chemical itself and its primary metabolites of concern are naturally present in humans – a fact that pre- supposes a certain level of these substances poses no toxic risk [15]. In this paper, the authors propose a level of methanol intake that is of insignificant risk to humans, in that it causes no increase in the most toxic metabolite (formic acid) above the normal background levels found in humans. That intake level is a single oral exposure to methanol of 20 mg/kg body weight. This represents 1400 mg for an individual weighing 70 kg. To receive this amount of methanol from a wine containing 400 mg/L methanol (the maximum methanol limit recommended by OIV for red wines – see Section 7), that individual would need to consume 3.5 litres (almost five 750 mL bottles) of wine in a single oral exposure.
According to the European Union Scientific Committee on Food, a healthy individual can metabolize 1500 mg per hour of methanol without showing any ill effects [16]. Note that 1500mg of methanol is approximately the amount of methanol contained in 3.75 litres (five 750 mL bottles) of a wine with a methanol content of 400mg/L (the maximum methanol limit recommended by OIV for red wines – see Sect. 7).This volume of wine would have to be consumed in one hour to ingest more methanol than a healthy individual could metabolise with no ill effects.
The Food and Drug Administration of the United States of America (FDA) reported that the No Observed Adverse Effect Level (NOAEL) in humans for methanol is 71 to 84 mg/kg body weight (bw)/day. Because this NOAEL was derived from studies in humans, the FDA developed an Acceptable Daily Intake (ADI) of 7.1 to 8.4 mg/kg bw/day by using a safety factor of 10 [17]. Note that an individual weighing 70 kg would have to consume about 1.25 litres of wine (almost two 750 mL bottles) a day with a methanol content of 400 mg/L (the maximum methanol limit recommended by OIV for red wines - see Sect. 7) to reach the low end of this ADI range.
7. Regulatory limits established for methanol levels in wine
Table 1 summarizes the limits set for methanol in wine in different global markets. It is clear that most economies and regions have established limits that take account of the levels of methanol typically found in wine, and make additional allowances for the possible use in production of exogenous pectinase enzymes and of DMDC, as mentioned earlier in this paper [18].
8. Purpose of methanol limits for wine
Comparing the toxicological information in Sect. 6 with the limits that have been established for methanol in wine (Sect. 7), it seems clear that the purpose of these limits is not to protect public health. This is because an individual weighing 70 kg would have to drink 1.25 litres of red wine a day containing the OIV recommended limit amount of methanol (400mg/L) in order to reach the lowest ADI value derived by the FDA (and including a safety factor of 10). According to the paper produced by the World Health Organization International Programme on Chemical Safety, a single oral exposure to 3.5 litres of this wine would not cause an increase in background levels of formic acid (the most toxic metabolite of methanol) in that consumer. Finally, the European Commission Scientific Committee on Food data suggests that the individual would have to consume 3.75 litres of the same wine in an hour to ingest more methanol than the 1500 mg that individuals can metabolize without ill effects.
If the methanol limits that have been established for wine do not represent a public health protection measure, it may be asked what purpose they actually serve. In practice, it seems possible that at one time they were intended to serve as an index of appropriate fruit handling in harvest and subsequent processing. In Sect. 3.1.1., the action of endogenous pectinases in forming methanol in wine was reviewed. Such enzymes will be liberated, and methanol generated, whenever fruit is damaged during its growth, its journey from the vineyard to the winery and in subsequent processing. It is recorded that fungal diseases that attack grapes in the vineyard most likely result in higher levels of methanol in the resulting juice and wine, and in Sect. 4 it was noted that late harvest wines (where fungal pressures will be more pronounced as the grapes remain longer in the vineyard) tend to have higher methanol contents [19]. Therefore, methanol content in wine may be expected to be higher where the fruit has been damaged either by moulds in the vineyard or by handling at harvest and during transportation to the winery.
The possibility of methanol limits historically serving as indices of appropriate fruit handling in wine production seems to be supported by the fact that many authorities differentiate between red wines on the one hand, and white wines and rosés on the other, establishing different methanol limits in each case. If the limits were purely set for toxicological reasons, there would be no need to do this; a single limit for methanol in wine would be appropriate regardless of whether it was white, rosé or red.
In Sect. 3 it was mentioned that the use of exogenous pectinase enzymes in wine production and also of DMDC as a pre-bottling sterilant are widely permitted, and that both of these processes, if used, will contribute somewhat to the total methanol content of wine (in recognition of which, governments and other appropriate intergovernmental bodies have increased their permissible limits for methanol in wine). It is clear, therefore, that there will no longer necessarily be such a clear relationship between a wine’s content of methanol and the fruit handling practices in the harvest and production of that wine.
As has been seen, even wine containing the maximum allowable methanol content by regulation never has a high enough concentration to give rise to public health concerns.
9. Conclusions
Methanol is produced quite naturally in wine by the action of endogenous pectinase enzymes on the grape pectins. The possible use of exogenous pectinase enzymes in the winery, as well as the possible treatment of the wine with dimethyldicarbonate just prior to bottling, will have the effect of increasing the methanol content of the wine to varying but small degrees. Many competent authorities around the world have chosen to establish limits for the methanol content of wine, and many have chosen to establish different limits for red wines compared with white and rosé. Yet in each case, when compared with toxicological data, it is clear that the limits do not really serve to protect public health, because the methanol content in wine does not become sufficiently high to even approach a safety concern.
At least two possible future directions are suggested by the observations in this paper:
First, there is no public health necessity for competent authorities to require methanol analysis of wine as a condition of market entry, since no additional consumer safety will be obtained by such measures. It is true that there have been rare wine adulteration incidents involving methanol in wine historically, but an extensive requirement for methanol analysis at the border seems an excessive and costly approach to mitigate the small possibility of such an event in international trade.
Second, if authorities determine that it is necessary to retain these limits, international trade could be greatly facilitated if they would consider taking the following steps:
-
Establishing limits that take account of the levels of methanol typically present in grape wines, and that also take account of the full range of permitted winemaking practices that may be applied during their production.
-
Given the wide variety of numerical values for existing methanol limits in wine, and of methods chosen
-
for the expression of those limits (as revealed in Table 1), considering the harmonisation of limits with those established by other competent authorities and/or appropriate intergovernmental bodies.
References
- A. L. Waterhouse, G. L. Sachs and D. W. Jeffery, Understanding Wine Chemistry, 54 (2016) [Google Scholar]
- Martindale: Extra Pharmacopoeia 25th Ed, 85–86 [Google Scholar]
- Data on wine production and pectinase usage. www.FIVS-Abridge.org (accessed on August 5, 2016) [Google Scholar]
- Data on wine production and DMDC usage. www.FIVS-Abridge.org (accessed on August 5, 2016) [Google Scholar]
- EFSA Panel on Food additives and Nutrient Sources added to Food (ANS), Scientific opinion on the re-evaluation of dimethyl dicarbonate (DMDC, E 242) as a food additive, 13(12), 4319, 11 (2015) [Google Scholar]
- W. R. Sponholz, Chemie des Weines. 385–411 (1989) [Google Scholar]
- B. Zoecklein, Wine Analysis and Production, 107 (1995) [Google Scholar]
- P Ribereau-Gayon, Handbook of Enology: The Chemistry of Wine Stabilization and Treatments 2nd Edition, 2 , 53 (2006) [Google Scholar]
- AWRI/Wine Australia Report, Typical Methanol Levels in Australian Wines (2016) [Google Scholar]
- C. Hou, Y. Lin, Y. Wang, C. Jiang, M. Wu, Journal of Food Composition and Analysis, Effect of storage conditions on methanol content of fruit and vegetable juices , 21 (5), 410–415 (2008) [Google Scholar]
- The Complete Technology Book on Alcoholic and Non-alcoholic Beverages (Fruit Juices, Whisky, Beer, Rum and Wine), 712 (2008) [Google Scholar]
- J. Clary, The Toxicology of Methanol, 48 (2013) [Google Scholar]
- Public Health England, Methanol: Toxicological Overview, 3 (2015) [Google Scholar]
- Public Health England, Methanol: Incident Management, 8 (2016) [Google Scholar]
- International Programme on Chemical Safety, Environmental Health Criteria, Methanol 196 (1997) http://www.inchem.org/documents/ehc/ehc/ehc196.htm (accessed August 5, 2016) [Google Scholar]
- European Commission Scientific Committee on Food, Opinion of the Scientific Committee on Food on the use of dimethyl dicarbonate (DMDC) in wines, (2001) [Google Scholar]
- Federal Register 58, no 204, 6088–6091, (1993) [Google Scholar]
- www.FIVS-Abridge.org (accessed on August 5, 2016) [Google Scholar]
- M. Amerine, M. Joslyn, Table Wines: The Technology of Their Production, 435 (1970) [Google Scholar]
All Tables
All Figures
 |
Figure 2. Comparison of MeOH concentrations in varietal wines |
| In the text | |
 |
Figure 3. Comparison of MeOH concentrations in vintage wines. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.




