| Issue |
BIO Web Conf.
Volume 68, 2023
44th World Congress of Vine and Wine
|
|
|---|---|---|
| Article Number | 02031 | |
| Number of page(s) | 7 | |
| Section | Oenology | |
| DOI | https://doi.org/10.1051/bioconf/20236802031 | |
| Published online | 06 December 2023 | |
Comparison of the influence of Saccharomyces pastorianus to Saccharomyces cerevisiae and Saccharomyces bayanus inoculation ratio to oenological characteristics of Sauvignon Blanc wine
1 Innovino Research & Development, 21 Meg. Alexandrou, 15351 Pallini, Greece
2 Department of Wine, Vine and Beverage Sciences, School of Food Science, University of West Attica, Ag. Spyridonos str, Egaleo, 12243 Athens, Greece
3 Fermentis, division of S.I. Lesaffre, 137 rue Gabriel Péri, 59703 Marcq-En-Baroeul, France
4 Laboratory of Enology & Alcoholic Drinks (LEAD), Department of Food Science & Human Nutrition, Agricultural University of Athens, 75 Iera Odos, 11855 Athens, Greece
The aim of our work was to evaluate the impact of different fermentation schemes by using mixed and pure cultures of S. pastorianus and S. cerevisiae or S. bayanus. For the mixed fermentation schemes, one strain of S. pastorianus has been inoculated under different proportions (99%/1%, 97%/3%, 95%/5%, 90%/10% and 70%/30% w/w) in co-inoculation with two commercial strains of S. cerevisiae and one commercial Saccharomyces bayanus strain. The fermentation kinetics has been controlled by density measurement and classical oenological analyses were performed based on OIV analytical methods. The population dynamics was evaluated by the specific interdelta PCR reaction of the Saccharomyces species in the beginning and in the end of the fermentation process. Volatile compounds of the wine aroma, such as esters, higher alcohols and thiols were analyzed by GC/MS. Sensory assessment by trained panel was carried out for all wines. The wines fermented with S. pastorianus, either in pure or mixed cultures, were characterized by significantly lower acetic acid production and higher malic acid degradation when compared to the wines fermented with S. cerevisiae strains. The presence of S. pastorianus strain enhanced the production of the varietal thiols when compared to the samples fermented with the S. cerevisiae pure cultures. The wines produced by the S. bayanus or cerevisiae monocultures and the ones produced by the co-culture with S. pastorianus at 70%-30% ratio (S. pastorianus to S. bayanus or cerevisiae) were overall better rated from a sensory point of view.
© The Authors, published by EDP Sciences, 2023
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Sauvignon Blanc wines are characterized by a very wide palette of flavors. Among the most distinctive ones are grapefruit, passion fruit and box tree notes, the occurrence of which has been attributed mainly to the presence of aromatic volatile thiols, namely 3-mercaptohexan-1-ol (3-MH), 3-mercaptohexyl acetate (3-MHA) and 4-mercapto-4-methylpentan-2-one (4-MMP) [1,2]. Their presence and concomitantly the intensity of the respective aromas seems to be largely dependent upon the yeast strain carrying out alcoholic fermentation [3,4].
In recent years, a consumer trend towards increased aromatic complexity and modified varietal typicity has been noted [5,6]. Research has initially addressed this demand through the use of non-Saccharomyces yeasts in combination with S. cerevisiae. However, such coexistence is not always feasible due to the antagonistic relationship that may be developed [3,7,8]. Interestingly, such an issue has not yet been reported when strains within the Saccharomyces sensu stricto group are combined.
S. pastorianus, a natural hybrid of S. cerevisiae and S. eubayanus mainly used for Lager beer production and the so-called “Prise De Mousse” yeasts S. bayanus (now grouped into the species S. cerevisiae but distinguished into its galactose negative sub-group) are interesting alternatives that have already been successfully employed to modulate Sauvignon Blanc typicity [9].
Therefore, the aim of the present study was to further assess the effect of the inoculation ratio between S. pastorianus and S. bayanus or S. cerevisiae on the oenological properties and aroma profile of Sauvignon Blanc wines. We studied these interactions by comparing pure and mixed cultures of the Saccharomyces species under different inoculation ratios. One S. pastorianus strain was tested under five co-inoculation combinations schemes with two S. cerevisiae and one S. bayanus commercial strains in a Sauvignon blanc must. For all fermentation trials, the population dynamics of inoculated and indigenous yeasts, the aromatic compounds with high oenological interest and the sensory profile of the produced wines were analyzed.
2 Materials and methods
2.1 Yeast strains and culture conditions
One strain of S. pastorianus SP2 under industrial development, one commercial strain of S. bayanus SafŒno™ BC S103 (Fermentis, France) and two commercial strains of S. cerevisiae SafŒno™ CK S102 (Fermentis, France) and SafŒno™ SH 12 (Fermentis, France) preconized for Sauvignon blanc wines and characterized by intensifying their aromatic profiles, were chosen for this study (Table 1). All strains were inoculated as active dry yeasts according to their population (CFU/g) in order to get the right inoculum ratio. Cell activation took place at 30 °C for 20 minutes; cell viability and population was verified for each inoculum by plate count in YPDA medium (20 g/L glucose, 10 g/L yeast extract, 10 g/L peptone, and 25 g/L agar). The inoculum level of pure and mixed cultures was approximately 106 CFU/mL.
Saccharomyces yeast species in pure and mixed cultures under different inoculation ratio.
2.2 Must preparation and fermentation kinetics
Grape must of Sauvignon blanc grapes (Asprokampos Nemea, Greece), with 12 °Be initial density and total acidity of 6 g tartaric acid/L was supplemented with sulfites (50 mg/L), clarified by cold treatment for 12 hours and decanted to the fermenters made of glass (30 L). Yeasts were inoculated as pure and mixed cultures at approximately 106 CFU/mL final concentration. The different inoculation ratio in mixed cultures were achieved by weighting different proportions of the dry yeast cells. 24 hours after yeast addition, 200 mg/L of organic nutrient (SpringFerm™, Fermentis, France) was added. Alcoholic fermentations were performed at 18 °C. During fermentation, samples were taken aseptically at 24 hours intervals for further analyses, while sugar depletion (Glucose<2 g/L) signified the end of the process.
2.3 Microbiological analyses and yeast molecular identification
Samples from all fermenters were taken every 48 hours for enumeration of the yeast population, while after 48 hours of yeast inoculation, in the middle and at the end of fermentation for molecular typing. From each fermenter, 1 mL sample per time point was taken to study the microbial growth. A sample taken from a fermenter without yeast inoculation was used as negative control during the whole fermentation procedure. Each sample was serially diluted in sterile saline. Measurement of Saccharomyces yeast and indigenous non-Saccharomyces yeast population levels was accomplished by plating serial dilutions on Wallerstein Laboratory Nutrient agar (WLN) or Lysine medium agar, respectively. All samples grown in WLN medium were plated in duplicate, with one plate incubated at 37 oC for 24 hours and the other at 28 oC for 48 hours, as S. pastorianus cells are thermosensitive and cannot grow significantly at temperatures above 30 oC. Incubation of Lysine agar plates was performed at 28 oC for 48-72 hours. The cell growth of total and wild yeast populations was measured as colony forming units (CFU/mL). After colonies enumeration of each plate, WLN plates that incubated at 28 oC were stored at 4 oC, in order to be used for yeasts genetic fingerprint analysis.
DNA fingerprinting took place after 48 hours, in the middle and in the end of the fermentation process in order to confirm that the dominated strains of the Saccharomyces species were the inoculated and not the indigenous ones. Saccharomyces strains that were responsible for the fermentation in each fermenter were identified by interdelta-sequence profile analysis using δ12 (5΄-TCAACAATGGAATCCCAAC-3΄) and δ2 (5΄-GTGGATTTTTATTCCAACA-3΄) primers [7,13]. A single colony was picked from the WLN plate of interest (the one incubated at 28 oC and stored at 4 oC) and the colony cells were resuspended in 20 μL of 0.02 N NaOH. Then, the cells were lysed through heat, after 10 minutes incubation at 98 oC in their resuspension solution, and 3 μL of lysed cells were used as template for each PCR reaction. A total of 16 colonies per fermenter and per time point were tested through PCR reaction with δ12/δ2 primer set, as well as 16 colonies from the negative control fermenter. Each PCR reaction was performed in 25 μL, containing 1 U of KapaTaq polymerase, 1xBuffer B, 1 mM MgCl2 (final concentration of MgCl2 at 2.5 mM), 0.2 mM dNTPs, and 800 nM of each primer. The PCR program was carried out as follows: 4 min at 94 oC, 35 cycles of 30 sec at 94 oC, 30 sec at 49 oC and 60 sec at 72 oC, followed by a final 10 minutes extension step at 72 °C. The PCR results were obtained by separation on 2% agarose gel containing 0.01% Midori Green in 1xSB buffer (5 mM Sodium borate decahydrate) and visualization under UV light. The agarose gels were run at 120 V for 35 minutes in 1xSB buffer. The resulting DNA profiles were compared to the respective of each strain employed. For the preparation of the latter, cells of the starter cultures (Sp2, CK S102, SH 12 or BC S103) were resuspended in water at 100 mg/mL concentration and serial dilutions were plated on WLN plates and incubated at 28 oC for 24-48 hours. Single colonies from the control cultures were used as template for the positive control reactions in PCR analysis.
2.4 Chemical analyses
2.4.1 Analysis of classical oenological parameters
The must was analyzed immediately after crushing of the grapes for the following parameters: glucose/fructose, total acidity, pH, malic acid, Yeast Assimilable Nitrogen (YAN), using enzymatic kits adapted for a Y15 Biosystems auto-analyser (Barcelona, Spain), while free and total SO2 were determined by titrimetric methods. Fermentations were monitored by daily enzymatic measurements of glucose and fructose [10]. The alcohol content of the wines was analyzed by NIR spectrometry.
2.4.2 Higher alcohols, acetates and esters quantification
Major and minor fermentation volatile compounds of wine aroma were analyzed by GC–MS using the Head-Space Solid Phase Micro Extraction (HS-SPME) procedure [7]. 25 mL of wine were spiked with the internal standard, 25 μg of 3-octanol (1 g/L) in a 40mL vial, then supplemented with 3 g of NaCl, a magnetic stir bar and then sealed with a screw-top cap with a silicon septum. The vial was placed on a heating stir plate and the samples were equilibrated by stirring at 750 rpm for 10 min at 40 °C. The SPME needle was inserted through the septum and the DVB/CAR/PDMS, 75 μm fiber was used to absorb the volatile compounds of the headspace for 30 min at 40 °C. The SPME needle was removed from the vial and inserted into the injector of GC for thermal desorption for 10 min. Analysis was performed using an Agilent 7890A GC, equipped with an Agilent 5873C MS detector. The column used was an DBWAX capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) and the gas carrier was helium with a flow rate of 1.2 mL/min. The injector and MS-transfer line were maintained at 250 °C and 260 °C, respectively. Oven temperature was held at 30 °C for 5 min and raised to 220 °C at 4 °C/min and held at this temperature for 20 min. The selective ion monitoring (SIM) mode was applied and as quantifiers ions were used ethyl isobutyrate (m/z 88), ethyl butyrate (m/z 71), ethyl 2 methyl butyrate(m/z 102), isoamyl acetate (m/z 87), isobutyl acetate (m/z 116), 3-octanol(m/z 59), ethyl hexanoate (m/z 88), ethyl octanoate (m/z 88), ethyl decanoate (m/z 157), 2 phenyl ethyl acetate (m/z 104), hexyl acetate, (m/z 69), isoamyl alcohol (m/z 87),2-methyl-1-propanol (m/z 87), hexanol-1 (m/z 84), 2-phenylethanol (m/z 122), 3-(methylthio)-1-propanol (m/z 96).
2.4.3 Varietal thiols quantification
Varietal thiols, 3-mercaptohexan-1-ol (3-MH), 3-mercaptohexyl acetate (3-MHA) 4-methyl-4-methylpentan-2-one (4-MMP), were quantified using the method described by Tominaga et al. [11].
2.5 Sensory analyses
The sensory assessment was done by a group of 8 trained judges with previous experience. Panelists were instructed to avoid, eating, drinking, and smoking 1 hour prior to the sessions. Panelists attended these sessions over a period of 1 months, twice per week. Training consisted of smelling of standard odors and description of wines. After 2 weeks the panelists chose some attributes characterizing the wines. During the following sessions the panelists were trained using appropriate solutions. The panelists were provided with 30 mL of samples in ISO wine glasses, coded with random three-digit numbers at room temperature (18-20 °C). The intensity of the sensory attributes examined was evaluated using a 10-point scale (1: null; 10: very strong).
3 Results and Discussion
3.1 Fermentation kinetics
Alcoholic fermentations of Sauvignon blanc clarified must were conducted in laboratory scale under different inoculation schemes by using Saccharomyces strains in pure and mixed cultures. Monocultures consisted of S. pastorianus strain (Sp2), two S. cerevisiae strains (Sc1 and Sc2) and one S. bayanus (Sb) strain. The co-inoculation of the Saccharomyces strains took place under different inoculation ratios (70%-30%, 80%-20%, 90%-10%, 95%-5% and 99%-1% w/w) of Sp2:Sc1 or Sc2 or Sb (Fig. 1). Fermentation kinetics were followed by sugar consumption while the yeast population dynamics by plate counting combined with molecular methods, which were performed in order to discriminate the inoculated from the indigenous yeast strains.
All fermentation trials lead to complete sugar consumption between 8 and 13 days.
 |
Figure 1 Fermentation kinetics of Sauvignon blanc must inoculated with pure and mixed cultures of Saccharomyces species. Values are means of two independent experiments. A, B and C, respectively Sb, Sc1 and Sc2 fermentations. |
3.2 Microbiological analyses
A total of 250 colonies were randomly selected from the agar plates used for enumeration of the S. spp strains and subjected to inter-delta fingerprinting in order to verify their identity. The inability of the S. pastorianus strain to grow adequately at 37 oC was verified in all cases; therefore, the temperature-based differentiation that was employed in the present study was accurate.
In all cases, alcoholic fermentation was driven by Saccharomyces yeasts while the non-Saccharomyces kept a low population along the fermentation process (data not shown).
Most interesting is to assess the results got on the proportion of S. pastorianus vs S. cerevisiae or bayanus as presented in Fig. 2. Whereas the percentage of S. pastorianus differed a bit from a PCR determination on 16 clones or from the differentiation of growth at 37°C and room temperature, we can observe that the S. bayanus allowed the S. pastorianus to survive much more than with the S. cerevisiae during fermentation showing a much better compatibility towards each other’s.
 |
Figure 2 Comparison of the percentages of implantation of Sp2 at different timings of the fermentation by PCR interdelta or growth test (Room temperature vs 37°C incubation). A, B, C respectively at 48hrs, 2/3rd of the alcoholic fermentation and at the end of the fermentation. |
3.3 Oenological analyses
In Table 2, some standard enological parameters after the completion of alcoholic fermentation, are presented. It is noticeable that the wines made with S. pastorianus and S. bayanus were the ones the most degrading the malic acid and a good correlation between the ratio of S. pastorianus was observed in the modulation of the content of the malic acid degraded.
In terms of acetic acid production, we confirmed the fact that S. pastorianus was one of the lowest producers, thus impacting much more the final volatile acidity of mixtures with medium producers as S. cerevisiae than S. bayanus, already a very low producer. So as for the production of SO2 but in reverse way, S. pastorianus being a strong producer while weakly resistant. No free SO2 was detected in any wine. In all cases, the ethanol produced ranged between 12.5 and 12.8 %vol.
The acidity and SO2 levels could thus explain partly the results of implantation we got for the S. pastorianus as mentioned in the paragraph 3.2. Moreover, the good correlations between the theoretical ratio of S. pastorianus and other species observed from an analytical point of view compared to the quite poor microbiological correlations obtained could as well be explained by the fact that the flocculation and the sedimentation of S. pastorianus is known to be much higher than for other species (data not shown).
Standard oenological parameters at the beginning and the end of alcoholic fermentation of Sauvignon Blanc must. 1Initial malic acid value was 1.89. 2Before yeast inoculation total SO2 and free SO2 were 24 and 11.5 mg/L, respectively. *Repetition of the values for the second fermentation made with Sp2 for better visualization.
3.4 Volatile compounds analyses
Quantification of thiols, higher alcohols and esters was performed for all the fermentation trials and showed on Fig. 3. The wine produced by spontaneous fermentation presented significant differences from the ones produced by inoculation.
3-MH, 3-MHA and 4-MMP, intrinsic compounds of Sauvignon blanc variety were quantified. The presence of Sc2 strain significantly increased the production of all respective thiols both in monoculture and co-inoculation conditions, and especially for 4-MMP, confirming its homozygosity on the expression of the long version of the IRC7 gene (IRC7L/IRC7L). Unexpectedly, for all strains of S. cerevisiae or bayanus, the conditions exhibiting the highest thiols production was when inoculated with Sp2 at a ratio of 70%-30% (Sp2/Sc1-Sc2-Sb), especially driven by the highest level of 3-MH production, hypothesizing a synergetical competition towards these aromatic compounds.
All acetates analyzed, and especially isoamyl acetate, known for contributing to wines the characteristic banana aroma was increased in all the co-inoculation schemes of Sc1 and Sp2 compared to the other fermentation conditions. It was a reverse effect with Sb (drastically) and with Sc2. The impact of inoculation modes on main ethyl esters (C4, C6, C8, C10) was less remarkable.
 |
Figure 3 Major volatile compounds at the end of alcoholic fermentation of Sauvignon Blanc must depending on the inoculation mode. |
3.5 Sensory analysis
In Fig. 4, the sensory analysis of the wines produced from the Sauvignon Blanc variety is presented. The different inoculation strategies affected the sensory analysis of the wines, to some extent.
The wines produced with the monocultures of the strains under study received different grades in the overall quality and the olfactory descriptor ‘aroma intensity’. More specifically, the grades received by the wine made with the monoculture of the S. bayanus Sb were higher than the respective received by the wine made with the monoculture of the S. pastorianus Sp2. Co-inoculation with both strains had also significant effect on the sensory analysis of the wines. More specifically, the olfactory descriptor ‘aroma intensity’ of the wine made with the inoculum ratio 90/10 and the overall acceptance of the wines made with the inoculum ratios 70/30 and 90/10 were improved compared to the wine made by the monoculture of the S. pastorianus Sp2. On the other hand, the olfactory descriptors ‘aroma intensity’, amylic’ and ‘complexity’ as well as the gustatory descriptors ‘body/roundness’ and ‘aftertaste/persistence’ and the overall quality of the wine made with the inoculum ratio 99/1 received lower grades than the respective made with the monoculture of the S. bayanus. Similarly, the wine made with the inoculum ratio 99/1 received higher grade in the olfactory descriptor ‘reduction’ compared to the one made with the monoculture of the S. bayanus (data not shown).
For the strain combination Sc1 and Sp2, the produced Sauvignon blanc wines that presented an overall better sensory profile characterized by higher complexity, balance, tropical fruit aromas and aromatic intensity were the ones fermented with the ratio Sp2 95%-Sc1 5% compared to the other inoculation ratio tested. When the Sauvignon blanc must was inoculated with the mixed cultures of Sp2 and Sc2 the fermentation scheme with preferred sensory profile was when Sp2 was co- inoculated at 70% with Sc2 30%. Under this inoculation scheme the produced wines were more floral, fruity and complex.
The sensory descriptors correlated to wine overall quality such as Global Mark, Balance and Complexity are distinguishable and seem to be related to either isoamyl acetate production for Sb and Sc1 or thiol production for Sc2. The wine fermented by the monoculture of Sc2 strain was indeed characterized by Tropical fruits aromas and a high Aromatic intensity, thus preferred by tasters as more related to Sauvignon Blanc typicality.
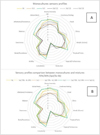 |
Figure 4 Comparison of the sensory profiles respectively of the monocultures and their mixtures with 70% of Sp2. A and B, respectively monocultures between themselves and monocultures with related mixtures. |
4 Conclusions
The present study aimed to assess the effect of different S. pastorianus and S. bayanus or S. cerevisiae inoculation ratios to the sensory profile of Sauvignon Blanc wines, towards modification of the varietal typicality. Relatively more days were necessary in order to achieve alcoholic fermentation completion with S. pastorianus in comparison to S. cerevisiae, S. bayanus and mixtures. Despite this delay, the alcohol level produced was same to the other species. The compatibility of the strains employed was different according to species but overall, the combination of a lower acidity (higher malic acid degradation) and SO2 production by the S. bayanus seemed to favor the persistence of the S. pastorianus along the fermentation, whereas the co-inoculation with S. cerevisiae was a bit more critical to the survival of the S. pastorianus. The oenological analyses revealed that the wine produced with the presence of S. pastorianus had lower volatile acidity compared to the wines fermented with pure cultures of S. cerevisiae, making this species of interest for winemaking. Malic acid degradation was also a strong point as the conversion of malic acid was up to 40%. For volatile components analyzed, both S. cerevisiae and S. bayanus strains used and the inoculation ratio significantly influenced the aroma profile. From a global point of view, the co-inoculated scheme Sp2 70% - Sc or Sb 30% was the one that resulted to Sauvignon blanc wine mostly preferred by the sensory panel and the one characterized by the highest thiols concentration. It would be interesting in the future to test this inoculation scheme in order to ferment more Sauvignon blanc must from regions with different geographical origin.
Overall S. pastorianus could be an interesting alternative for wine making and thus further research should be devoted to these species.
References
- Nakao, Y., Kanamori, T., Itoh, T., Kodama, Y., Rainieri, S., Nakamura, N., et al. (2009). Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 16, 115–129, doi: 10.1093/dnares/dsp003 [CrossRef] [PubMed] [Google Scholar]
- Libkind, D., Hittinger, C.T., Valerio, E., Goncalves, C., Dover, J., Johnston, M., et al. (2011). Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. U.S.A. 108, 14539–14544 [CrossRef] [PubMed] [Google Scholar]
- Ribereau Gayon Handbook of enology. Volume 1, The microbiology of wine and vinifications, Ed John Wiley 2006 [Google Scholar]
- Meier-Dörnberg, T. Genetic and phenotypic characterization of different top-fermenting Saccharomyces cerevisiae ale yeast isolates. In Proceedings of the 36th European Brewery Convention, Ljubljana, Slovenia, 14–18 May 2017 [Google Scholar]
- Castellari L., Ferruzi, M., Magrini, A. Giudici, P., et al., 1994: Unbalanced wine fermentation by cryotolerant vs non-cryotolerant Saccharomyces strains. Vitis 33, 49–52 [Google Scholar]
- Massoutier C., Alexandre H., Feuillat M., Charpentier C., 1998: Isolation and characterization of cryotolerant Saccharomyces strains. Vitis 37(1), 55–59 [Google Scholar]
- Nurgel, C., & Pickering, G. (2005) Contribution of glycerol, ethanol and sugar to the perception of viscosity and density elicited by model white wines. Journal of Texture Studies 36, 303–323 [CrossRef] [Google Scholar]
- Petitgonnet, C., Klein, G.L., Roullier-Gall, C., Schmitt-Kopplin, P., Quintanilla-Casas, B., Vichi, S., Julien-David, D., Alexandre, H. Influence of cell-cell contact between L. thermotolerans and S. cerevisiae on yeast interactions and the exo-metabolome. Food Microbiol. 2019, 83, 122–133 [CrossRef] [Google Scholar]
- Dimopoulou, M., Troianou, V., Toumpeki, C., Gosselin, Y., Dorignac, É., Kotseridis, Y. Effect of strains from different Saccharomyces species used in different inoculation schemes on chemical composition and sensory characteristics of Sauvignon blanc wine. OENO One 2020 54, 745–759 [CrossRef] [Google Scholar]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis, OIV: Paris, France, 2020 [Google Scholar]
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of new volatile thiols in the aroma of Vitis vinifera L. var. Sauvignon blanc wines. Flavour Fragr. J. 1998, 13(3), 159–162 [CrossRef] [Google Scholar]
- Dimopoulou, M., Troianou, V., Toumpeki, C., Lola, D. Goulioti, E., Tzamourani, A., Dorignac, E., Paramithiotis, S., Kotseridis, Y. Influence of Saccharomyces pastorianus and Saccharomyces bayanus Inoculation Ratio to Oenological Characteristics of Sauvignon Blanc Wine. Appl. Sci. 2023 13, 3393 https://doi.org/10.3390/app13063393 [CrossRef] [Google Scholar]
- Dimopoulou, M., Goulioti, E., Troianou, V., Toumpeki, C., Paramithiotis, S., Gosselin, Y., Dorignac, E., Papadopoulos, G., Kotseridis, Y. Effect of Saccharomyces cerevisiae and Saccharomyces pastorianus Co-Inoculation on Alcoholic Fermentation Behavior and Aromatic Profile of Sauvignon Blanc Wine. Fermentation 2022 8, 539 https://doi.org/10.3390/fermentation8100539 [CrossRef] [Google Scholar]
All Tables
Saccharomyces yeast species in pure and mixed cultures under different inoculation ratio.
Standard oenological parameters at the beginning and the end of alcoholic fermentation of Sauvignon Blanc must. 1Initial malic acid value was 1.89. 2Before yeast inoculation total SO2 and free SO2 were 24 and 11.5 mg/L, respectively. *Repetition of the values for the second fermentation made with Sp2 for better visualization.
All Figures
 |
Figure 1 Fermentation kinetics of Sauvignon blanc must inoculated with pure and mixed cultures of Saccharomyces species. Values are means of two independent experiments. A, B and C, respectively Sb, Sc1 and Sc2 fermentations. |
| In the text | |
 |
Figure 2 Comparison of the percentages of implantation of Sp2 at different timings of the fermentation by PCR interdelta or growth test (Room temperature vs 37°C incubation). A, B, C respectively at 48hrs, 2/3rd of the alcoholic fermentation and at the end of the fermentation. |
| In the text | |
 |
Figure 3 Major volatile compounds at the end of alcoholic fermentation of Sauvignon Blanc must depending on the inoculation mode. |
| In the text | |
 |
Figure 4 Comparison of the sensory profiles respectively of the monocultures and their mixtures with 70% of Sp2. A and B, respectively monocultures between themselves and monocultures with related mixtures. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.



